Drug Class Brand Or Label Name* Generic Name
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Selective Mtorc2 Inhibitor Therapeutically Blocks Breast Cancer Cell Growth and Survival
Author Manuscript Published OnlineFirst on January 22, 2018; DOI: 10.1158/0008-5472.CAN-17-2388 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Selective mTORC2 inhibitor therapeutically blocks breast cancer cell growth and survival Thomas A. Werfel1, 3, Shan Wang2, Meredith A. Jackson1, Taylor E. Kavanaugh1, Meghan Morrison Joly3, Linus H. Lee1, Donna J. Hicks3, Violeta Sanchez4, Paula Gonzalez Ericsson4, Kameron V. Kilchrist1, Somtochukwu C. Dimobi1, Samantha M. Sarett1, Dana Brantley-Sieders2, Rebecca S. Cook1,3,4* and Craig L. Duvall1* 1Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232 USA 2Department of Medicine, Vanderbilt University Medical Center, Nashville, TN 37232.USA 3Department of Cell and Developmental Biology, Vanderbilt University School of Medicine, Nashville, TN 37232 USA 4Breast Cancer Research Program, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN 37232 USA Running Title: A selective mTORC2 inhibitor blocks breast cancer growth Key Words: Breast Cancer, mTOR, Rictor, RNA interference, Nanomedicine *To whom correspondence should be addressed: Craig L. Duvall, PhD Vanderbilt University School of Engineering Department of Biomedical Engineering Nashville, TN 37232 Phone: (615) 322-3598 Fax: (615) 343-7919 Email: [email protected] Rebecca S. Cook, PhD Vanderbilt University Medical Center Department of Cancer Biology Nashville, TN 37232 Phone: (615) 936-3813 Fax: (615) 936-3811 Email: [email protected] Funding. This work was supported by Specialized Program of Research Excellence (SPORE) grant NIH P50 CA098131 (VICC), Cancer Center Support grant NIH P30 CA68485 (VICC), NIH F31 CA195989-01 (MMW), NIH R01 EB019409, DOD CDMRP OR130302, NSF GFRP 1445197, and CTSA UL1TR000445 from the National Center for Advancing Translational Sciences. -

Angiotensin-Converting Enzyme (ACE) Inhibitors Single Entity Agents
Therapeutic Class Overview Angiotensin-Converting Enzyme (ACE) Inhibitors Single Entity Agents Therapeutic Class Overview/Summary: The renin-angiotensin-aldosterone system (RAAS) is the most important component in the homeostatic regulation of blood pressure.1,2 Excessive activity of the RAAS may lead to hypertension and disorders of fluid and electrolyte imbalance.3 Renin catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin I is then cleaved to angiotensin II by angiotensin- converting enzyme (ACE). Angiotensin II may also be generated through other pathways (angiotensin I convertase).1 Angiotensin II can increase blood pressure by direct vasoconstriction and through actions on the brain and autonomic nervous system.1,3 In addition, angiotensin II stimulates aldosterone synthesis from the adrenal cortex, leading to sodium and water reabsorption. Angiotensin II exerts other detrimental cardiovascular effects including ventricular hypertrophy, remodeling and myocyte apoptosis.1,2 The ACE inhibitors block the conversion of angiotensin I to angiotensin II, and also inhibit the breakdown of bradykinin, a potent vasodilator.4 Evidence-based guidelines recognize the important role that ACE inhibitors play in the treatment of hypertension and other cardiovascular and renal diseases. With the exception of Epaned® (enalapril solution) and Qbrelis® (lisinopril solution), all of the ACE inhibitors are available generically. Table 1. Current Medications Available in Therapeutic Class5-19 Generic Food and Drug Administration -

An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: a Review
Muthadi Radhika Reddy /J. Pharm. Sci. & Res. Vol. 12(7), 2020, 925-940 An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review Muthadi Radhika Reddy1* 1School of pharmacy, Gurunanak Institute of Technical Campus, Hyderabad, Telangana, India and Department of Pharmacy, Gandhi Institute of Technology and Management University, Vizag, Andhra Pradesh, India INTRODUCTION 2. Useful in situations where rapid onset of action Fast dissolving drug delivery systems were first developed required such as in motion sickness, allergic attack, in the late 1970s as an alternative to conventional dosage coughing or asthma forms. These systems consist of solid dosage forms that 3. Has wide range of applications in pharmaceuticals, Rx disintegrate and dissolve quickly in the oral cavity without Prescriptions and OTC medications for treating pain, the need of water [1]. Fast dissolving drug delivery cough/cold, gastro-esophageal reflux disease,erectile systems include orally disintegrating tablets (ODTs) and dysfunction, sleep disorders, dietary supplements, etc oral thin films (OTFs). The Centre for Drug Evaluation [4] and Research (CDER) defines ODTs as,“a solid dosage 4. No water is required for the administration and hence form containing medicinal substances which disintegrates suitable during travelling rapidly, usually within a matter of seconds, when placed 5. Some drugs are absorbed from the mouth, pharynx upon the tongue” [2]. USFDA defines OTFs as, “a thin, and esophagus as the saliva passes down into the flexible, non-friable polymeric film strip containing one or stomach, enhancing bioavailability of drugs more dispersed active pharmaceutical ingredients which is 6. May offer improved bioavailability for poorly water intended to be placed on the tongue for rapid soluble drugs by offering large surface area as it disintegration or dissolution in the saliva prior to disintegrates and dissolves rapidly swallowing for delivery into the gastrointestinal tract” [3]. -

Comprehensive Review on Herbal Toothpaste
Annals of R.S.C.B., ISSN:1583-6258, Vol. 25, Issue 4, 2021, Pages. 9509 - 9518 Received 05 March 2021; Accepted 01 April 2021. Comprehensive Review on Herbal Toothpaste Divya S1, Dr. J Suresh1*, Dr. S. Meenakshi 2 1. Department of Pharmacognosy, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru-570015 2.Department of Prosthodontics, JSS Dental College, JSS Academy of Higher Education &Research, Mysuru-570015 Corresponding author Dr. J. Suresh Professor Department of Pharmacognosy, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru-570015 Email: [email protected] Mobile No: 9480197611 ABSTRACT Herbal products for general as well as for oral health care have gained prominence around worldwide. People who aspire towards the use of herbal products often consider these products are relatively safer than products containing synthetic ingredients. Based on increased usage of herbal cosmetics we tried to make a comprehensive review on herbal toothpaste that helps to maintain a proper oral hygiene and free from periodontal disorder, reduce stain, gingivitis, calculus and caries. The present review gives basic information regarding antimicrobial potential of various herbs, formulationexcipients, that can be used in preparation of toothpaste. Key Words: Herbal toothpaste, Anti-microbial screening, Periodontal disorder, Gingivitis, calculus, Dental caries. INTRODUCTION In developing countries, the intensity of infections caused by certain pathogenic micro- organisms that may leads to mortality as well as morbidity in immune-suppressant patients [1]. Multiple abrasives, scent, green lead was used to remove the stain from teeth until mid- 19thcentury. In Medieval period rock salt, fine sand were the key ingredients used by Arabs for tooth cleaning.In the period 1950 AD, Dr. -

Chapter 1 Controlling Drug Delivery
chapter 1 Controlling drug delivery Overview In this chapter we will: & differentiate drug delivery systems according to their physical state & differentiate drug delivery systems according to their route of administration & differentiate drug delivery systems according to their type of drug release & discuss drug transport across epithelial barriers. Introduction KeyPoints & Continued developments in Pharmacotherapy can be defined as the treatment chemistry, molecular biology and prevention of illness and disease by means of and genomics support the drugs of chemical or biological origin. It ranks discovery and developments among the most important methods of medical of new drugs and new drug treatment, together with surgery, physical targets. & treatment, radiation and psychotherapy. There The drug delivery system are many success stories concerning the use of employed can control the pharmacological action of a drugs and vaccines in the treatment, prevention drug, influencing its and in some cases even eradication of diseases pharmacokinetic and (e.g. smallpox, which is currently the only subsequent therapeutic human infectious disease completely profile. eradicated). Although it is almost impossible to estimate the exact extent of the impact of pharmacotherapy on human health, there can be no doubt that pharmacotherapy, together with improved sanitation, better diet and better housing, has improved people’s health, life expectancy and quality of life. Tip Unprecedented developments in genomics Combinatorial chemistry is a way to and molecular biology today offer a plethora of build a variety of structurally related new drug targets. The use of modern chemical drug compounds rapidly and synthetic methods (such as combinatorial systematically. These are assembled chemistry) enables the syntheses of a large from a range of molecular entities number of new drug candidates in shorter times which are put together in different ‘ ’ than ever before. -

WO 2010/044736 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 22 April 2010 (22.04.2010) WO 2010/044736 Al (51) International Patent Classification: (74) Agents: Bratt, Henrik et al; Jarnvagsgatan 10 A, S-251 A61K 9/24 (2006.01) A61P 25/34 (2006.01) 10 Helsingborg (SE). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/SE2009/05 1163 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (22) Date: International Filing CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, 13 October 2009 (13.10.2009) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (26) Publication Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (30) Priority Data: NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, 0802 189-1 14 October 2008 (14.10.2008) SE SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant (for all designated States except US): McNeil AB [SE/SE]; Box 941, S-25 1 09 Helsingborg (SE). (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (72) Inventors; and GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (75) Inventors/Applicants (for US only): LINDELL, Katari- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, na [SE/SE]; Skarhult 1325, S-241 93 Eslδv (SE). -

Introduction to Hospital and Health-System Pharmacy Practice 59 Tients with a Specific Disease State Or for Activities Related to Self Governance Diagnosis
Part II: Managing Medication Use CHAPTER 4 Medication Management Kathy A. Chase ■■ ■■■ Key Terms and Definitions Learning Objectives ■■ Closed formulary: A list of medica- After completing this chapter, readers tions (formulary) which limits access should be able to: of a practitioner to some medications. 1. Describe the purpose of a formulary A closed formulary may limit drugs to system in managing medication use in specific physicians, patient care areas, or institutions. disease states via formulary restrictions. 2. Discuss the organization and role of the ■■ Drug formulary: A formulary is a pharmacy and therapeutics committee. continually updated list of medications 3. Explain how formulary management and related information, representing works. the clinical judgment of pharmacists, 4. List the principles of a sound formulary physicians, and other experts in the system. diagnosis and/or treatment of disease 5. Define key terms in formulary manage- and promotion of health. ment. ■■ Drug monograph: A written, unbi- ased evaluation of a specific medica- tion. This document includes the drug name, therapeutic class, pharmacology, indications for use, summary of clinical trials, pharmacokinetics/dynamics, ad- verse effects, drug interactions, dosage regimens, and cost. ■■ Drug therapy guidelines: A document describing the indications, dosage regi- mens, duration of therapy, mode(s) of administration, monitoring parameters and special considerations for use of a specific medication or medication class. ■■ Drug use evaluation (DUE): A process used to assess the appropriate- ness of drug therapy by engaging in the evaluation of data on drug use in a given health care environment against predetermined criteria and standards. ◆■ Diagnosis-related DUE: A drug use evaluation completed on pa- INTRODUCTION TO HOSPITAL AND HEALTH-SYSTEM PHARMACY PRACTICE 59 tients with a specific disease state or for activities related to self governance diagnosis. -

Utilization and Program Costs of Statins for Wisconsin Medicaid
To: Prescribing Physicians, Pharmacies From: Wisconsin Medicaid, Division of Health Care Financing January 2004 Utilization and Program Costs of Statins for Wisconsin Medicaid PRIOR AUTHORIZATION GUIDELINES (atorvastatin), Zocor (simvastatin), Pravachol (pravastatin), Crestor (rosuvastatin), Lescol (fluvastatin), and Lescol XL In order to encourage the use of generic lovastatin, the Wis- (fluvastatin XL). Products that contain an HMG-CoA reductase consin Medicaid program began requiring prior authorization inhibitor combined with another ingredient (e.g. Advicor) were for brand name HMG-CoA reductase inhibitors on April 15, not included in this analysis. 2003. Prior authorization was made available through the STAT-PA system. Only recipients new to statin drugs are re- The generic form of lovastatin is significantly less expen- quired to try lovastatin first. The criteria for determining prior sive to the Medicaid program than brand name products. authorization includes: Average cost to the Wisconsin Medicaid Program for generic 1 · Any recipient currently on an effective brand name statin lovastatin 40 mg is $1.20 per tablet and for a brand name will be granted PA to continue on that statin drug. HMG-CoA reductase inhibitors (including the brand name · Any recipient who requires >35% reduction in low-density forms of lovastatin) range from $1.65 to $4.18 per equipotent dosage2 (table 1). lipoprotein (LDL) cholesterol will be granted PA to start on the brand name statin drugs. Table I · Any recipient who has impaired renal function will be Cost Per Tablet for Wisconsin Medicaid granted PA to start on the brand name statin drugs. · Any recipient who is at high risk for drug interactions will be granted PA to start on the brand name statin drugs. -
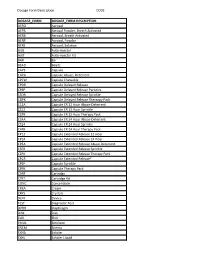
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Formulation and Evaluation of Liposomal Drug Delivery System for Doxorubicin Hydrochloride
FORMULATION AND EVALUATION OF LIPOSOMAL DRUG DELIVERY SYSTEM FOR DOXORUBICIN HYDROCHLORIDE A dissertation submitted to THE TAMILNADU Dr. M.G.R.MEDICAL UNIVERSITY, CHENNAI. In partial fulfillment of the requirements for the award of degree of MASTER OF PHARMACY IN PHARMACEUTICS BY REG.NO: 26091390 Under the Guidance of Prof. S.P.SENTHIL, M.Pharm., ( Ph.D.,) OCTOBER -2011 THE ERODE COLLEGE OF PHARMACY AND RESEARCH INSTITUTE ERODE -638112, TAMILNADU DEDICATED TO My Beloved Family, Teachers & Friends CERTIFICATES The Erode College Of Pharmacy and Research Institute Prof.S.P.SENTHIL, M.Pharm.,( Ph.D.,) Department of pharmaceutics, Perundurai Main Road, Veppampalayam, Erode-638112, India. e-mail : [email protected] CERTIFICATE This is to certify that the investigation in this thesis entitled “FORMULATION AND EVALUATION OF LIPOSOMAL DRUG DELIVERY SYSTEM FOR DOXORUBICIN HYDROCHLORIDE” submitted to The Tamilnadu Dr. M.G.R. Medical University Chennai. For partial fulfillment of the award of degree of Master of pharmacy in Pharmaceutics was carried out by Reg. No: 26091390 in the department of pharmaceutics, The Erode College of pharmacy, Erode, under my guidance and supervision This work is original and has not been submitted in part or full to any other degree or diploma of this or any other university. Place: Erode Prof. S.P.SENTHIL, M.Pharm.,(Ph.D.,) Date: The Erode College Of Pharmacy and Research Institute Dr.V.Ganesan, M.Pharm., Ph.D., Professor and HOD of Pharmaceutics, Perundurai Main Road, Veppampalayam, Erode-638112, India. CERTIFICATE This is to certify that the investigation in this thesis entitled “FORMULATION AND EVALUATION OF LIPOSOMAL DRUG DELIVERY SYSTEM FOR DOXORUBICIN HYDROCHLORIDE ” submitted to the Tamil Nadu Dr. -

Medicinal Cannabis Dosage Forms in California an Overview of Cannabis Dosage Forms
Mica Gross President Sante Botanica / COO MDBioLogics Medicinal Cannabis Dosage Forms in California An Overview of Cannabis Dosage Forms Forms of dosage are the specific vehicles by which cannabis, or cannabinoids, are delivered into the body. Cannabis (drug-containing plant) —> The “form” in which THC and other cannabinoids Extracted Oil (drug substance) —> are administered determines how they moves and Delivery / Intake (drug product) works medicinally inside the body: ie the pharmacokinetics of cannabis. Both the plant material and the oils yielded through the extraction process influence the performance of the different forms of intake, which each have a particular set of benefits. An Overview of Cannabis Dosage Forms The medicinal effect experienced by the end- user is a function of the quality of the raw material – the cannabinoid and terpene profile of the cannabis – and of the derived oil yielded during extraction. The goal is to preserve the fidelity of the volatile medicinal compounds – such as the cannabinoids, terpenes, and flavonoids – throughout the entire chain of custody so that the therapeutic effect is intact and the dose is delivered in the most efficacious means possible. An Overview of Cannabis Dosage Forms Each form of intake is formulated to take advantage of the pharmacokinetic pathways as effectively as possible in delivering a therapeutic dose of cannabinoids (e.g. THC or CBD). An Overview of Cannabis Dosage Forms Important factors when considering each form of intake: • Bioavailability – the fraction of the administered