Differentiating Lower Motor Neuron Syndromes
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Clinically Undetected Motor Neuron Disease in Pathologically Proven Frontotemporal Lobar Degeneration with Motor Neuron Disease
ORIGINAL CONTRIBUTION Clinically Undetected Motor Neuron Disease in Pathologically Proven Frontotemporal Lobar Degeneration With Motor Neuron Disease Keith A. Josephs, MST, MD; Joseph E. Parisi, MD; David S. Knopman, MD; Bradley F. Boeve, MD; Ronald C. Petersen, MD, PhD; Dennis W. Dickson, MD Background: Frontotemporal lobar degeneration with evidence of motor neuron disease. Semiquantitative motor neuron disease (FTLD-MND) is a pathological analysis of motor and extramotor pathological findings entity characterized by motor neuron degeneration and revealed a spectrum of pathological changes underlying frontotemporal lobar degeneration. The ability to detect FTLD-MND. Hippocampal sclerosis, predominantly of the clinical signs of dementia and motor neuron disease the subiculum, was a significantly more frequent occur- in pathologically confirmed FTLD-MND has not been rence in the cases without clinical evidence of motor assessed. neuron disease (PϽ.01). In addition, neuronal loss, gliosis, and corticospinal tract degeneration were less Objectives: To determine if all cases of pathologically severe in the other 3 cases without clinical evidence of confirmed FTLD-MND have clinical evidence of fronto- motor neuron disease. temporal dementia and motor neuron disease, and to de- termine the possible reasons for misdiagnosis. Conclusions: Clinical diagnostic sensitivity for the el- ements of FTLD-MND is modest and may be affected by Method: Review of historical records and semiquantita- the fact that FTLD-MND represents a spectrum of patho- tive analysis of the motor and extramotor pathological find- logical findings, rather than a single homogeneous en- ings of all cases of pathologically confirmed FTLD-MND. tity. Detection of signs of clinical motor neuron disease is also difficult when motor neuron degeneration is mild Results: From a total of 17 cases of pathologically con- and in patients with hippocampal sclerosis. -

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

Voice of the Patient Report for Spinal Muscular Atrophy
V OICE OF THE PATIENT REPORT A summary report resulting from an Externally-Led Patient Focused Drug Development Meeting reflecting the U.S. Food and Drug Administration (FDA) Patient-Focused Drug Development Initiative Spinal Muscular Atrophy (SMA) Externally Led Public Meeting: April 18, 2017 Report Date: January 10, 2018 Title of Resource: The Voice of the Patient Report for Spinal Muscular Atrophy Authors: Contributors to the collection of the information and development of the document are: Cure SMA: Rosangel Cruz, Megan Lenz, Lisa Belter, Kenneth Hobby, Jill Jarecki Medical Writer: Theo Smart Cruz, Lenz, Belter, Hobby, and Jarecki are all employees of Cure SMA and have no disclosures. Cure SMA has received funding from certain companies for work on projects unrelated to the Patient-Focused Drug Development meeting. Funding Received: The report was funded by grants received from the SMA Industry Collaboration to support Cure SMA’s production and execution of the Externally-Led Patient-Focused Drug Development initiative for SMA and the engagement of an outside medical writing professional to assist in the development, editing, and production of The Voice of the Patient report for SMA. The members of the SMA Industry Collaboration are Astellas Pharmaceuticals, AveXis, Inc., Biogen, Genentech/Roche Pharmaceuticals, Cytokinetics Inc., Novartis Pharmaceuticals, and Ionis Pharmaceuticals, Inc. Version Date: January 10, 2018 Revision Statement: This resource document has not been revised and/or modified in any way after January 10, 2018. Statement of Use: Cure SMA has the necessary permissions to submit the “The Voice of the Patient for SMA” report to the U.S. FDA. -
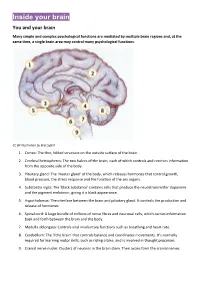
Inside Your Brain You and Your Brain
Inside your brain You and your brain Many simple and complex psychological functions are mediated by multiple brain regions and, at the same time, a single brain area may control many psychological functions. CC BY Illustration by Bret Syfert 1. Cortex: The thin, folded structure on the outside surface of the brain. 2. Cerebral hemispheres: The two halves of the brain, each of which controls and receives information from the opposite side of the body. 3. Pituitary gland: The ‘master gland’ of the body, which releases hormones that control growth, blood pressure, the stress response and the function of the sex organs. 4. Substantia nigra: The ‘black substance’ contains cells that produce the neurotransmitter dopamine and the pigment melatonin, giving it a black appearance. 5. Hypothalamus: The interface between the brain and pituitary gland. It controls the production and release of hormones. 6. Spinal cord: A large bundle of millions of nerve fibres and neuronal cells, which carries information back and forth between the brain and the body. 7. Medulla oblongata: Controls vital involuntary functions such as breathing and heart rate. 8. Cerebellum: The ‘little brain’ that controls balance and coordinates movements. It’s normally required for learning motor skills, such as riding a bike, and is involved in thought processes. 9. Cranial nerve nuclei: Clusters of neurons in the brain stem. Their axons form the cranial nerves. Your brain underpins who you are. It stores your knowledge and memories, gives you the capacity for thought and emotion, and enables you to control your body. The brain is just one part of the nervous system. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

ALS and Other Motor Neuron Diseases Can Represent Diagnostic Challenges
Review Article Address correspondence to Dr Ezgi Tiryaki, Hennepin ALS and Other Motor County Medical Center, Department of Neurology, 701 Park Avenue P5-200, Neuron Diseases Minneapolis, MN 55415, [email protected]. Ezgi Tiryaki, MD; Holli A. Horak, MD, FAAN Relationship Disclosure: Dr Tiryaki’s institution receives support from The ALS Association. Dr Horak’s ABSTRACT institution receives a grant from the Centers for Disease Purpose of Review: This review describes the most common motor neuron disease, Control and Prevention. ALS. It discusses the diagnosis and evaluation of ALS and the current understanding of its Unlabeled Use of pathophysiology, including new genetic underpinnings of the disease. This article also Products/Investigational covers other motor neuron diseases, reviews how to distinguish them from ALS, and Use Disclosure: Drs Tiryaki and Horak discuss discusses their pathophysiology. the unlabeled use of various Recent Findings: In this article, the spectrum of cognitive involvement in ALS, new concepts drugs for the symptomatic about protein synthesis pathology in the etiology of ALS, and new genetic associations will be management of ALS. * 2014, American Academy covered. This concept has changed over the past 3 to 4 years with the discovery of new of Neurology. genes and genetic processes that may trigger the disease. As of 2014, two-thirds of familial ALS and 10% of sporadic ALS can be explained by genetics. TAR DNA binding protein 43 kDa (TDP-43), for instance, has been shown to cause frontotemporal dementia as well as some cases of familial ALS, and is associated with frontotemporal dysfunction in ALS. Summary: The anterior horn cells control all voluntary movement: motor activity, res- piratory, speech, and swallowing functions are dependent upon signals from the anterior horn cells. -

Approach to a Patient with Hemiplegia and Monoplegia
CHAPTER Approach to a Patient with Hemiplegia and Monoplegia 27 Sudhir Kumar, Subhash Kaul INTRODUCTION 4. Injury to multiple cervical nerve roots. Monoplegia and hemiplegia are common neurological 5. Functional or psychogenic. symptoms in patients presenting to the emergency department as well as outpatient department. Insidious onset, gradually progressive monoplegia affecting lower limb can be caused by the following Monoplegia refers to weakness of one limb (either arm or conditions: leg) and hemiplegia refers to weakness of one arm and leg on the same side of body (either left or right side). 1. Tumor of the contralateral frontal lobe. There are a variety of underlying causes for monoplegia 2. Tumor of spinal cord at thoracic or lumbar level. and hemiplegia. The causes differ in different age groups. 3. Chronic infection of brain (frontal lobe) or spinal The causes also differ depending on the onset, progression cord (thoracic or lumbar level), such as tuberculous. and duration of weakness. Therefore, one needs to adopt a systematic approach during history taking and 4. Lumbosacral-plexopathy, due to diabetes mellitus. examination in order to arrive at the correct diagnosis. Insidious onset, gradually progressive monoplegia, Appropriate investigations after these would confirm the affecting upper limb, can be caused by one of the following diagnosis. conditions: The aim of this chapter is to systematically look at the 1. Tumor of the contralateral parietal lobe. differential diagnosis of monoplegia and hemiplegia and outline the approach needed to pinpoint the exact 2. Compressive lesion (tumor, large disc, etc) in underlying cause. cervical cord region. 3. Chronic infection of the brain (parietal lobe) or APPROACH TO THE DIAGNOSIS OF MONOPLEGIA spinal cord (cervical region), such as tuberculous. -

Neuromuscular Disorders Neurology in Practice: Series Editors: Robert A
Neuromuscular Disorders neurology in practice: series editors: robert a. gross, department of neurology, university of rochester medical center, rochester, ny, usa jonathan w. mink, department of neurology, university of rochester medical center,rochester, ny, usa Neuromuscular Disorders edited by Rabi N. Tawil, MD Professor of Neurology University of Rochester Medical Center Rochester, NY, USA Shannon Venance, MD, PhD, FRCPCP Associate Professor of Neurology The University of Western Ontario London, Ontario, Canada A John Wiley & Sons, Ltd., Publication This edition fi rst published 2011, ® 2011 by Blackwell Publishing Ltd Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing program has been merged with Wiley’s global Scientifi c, Technical and Medical business to form Wiley-Blackwell. Registered offi ce: John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial offi ces: 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 111 River Street, Hoboken, NJ 07030-5774, USA For details of our global editorial offi ces, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell The right of the author to be identifi ed as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. -

A System for Studying Mechanisms of Neuromuscular Junction Development and Maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R
© 2016. Published by The Company of Biologists Ltd | Development (2016) 143, 2464-2477 doi:10.1242/dev.130278 TECHNIQUES AND RESOURCES RESEARCH ARTICLE A system for studying mechanisms of neuromuscular junction development and maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R. Gomes1,3,*,‡ ABSTRACT different animal models and cell lines (Chen et al., 2014; Corti et al., The neuromuscular junction (NMJ), a cellular synapse between a 2012; Lenzi et al., 2015) with the hope of recapitulating some motor neuron and a skeletal muscle fiber, enables the translation of features of neuromuscular diseases and understanding the triggers chemical cues into physical activity. The development of this special of one of their common hallmarks: the disruption of the structure has been subject to numerous investigations, but its neuromuscular junction (NMJ). The NMJ is one of the most complexity renders in vivo studies particularly difficult to perform. studied synapses. It is formed of three key elements: the lower motor In vitro modeling of the neuromuscular junction represents a powerful neuron (the pre-synaptic compartment), the skeletal muscle (the tool to delineate fully the fine tuning of events that lead to subcellular post-synaptic compartment) and the Schwann cell (Sanes and specialization at the pre-synaptic and post-synaptic sites. Here, we Lichtman, 1999). The NMJ is formed in a step-wise manner describe a novel heterologous co-culture in vitro method using rat following a series of cues involving these three cellular components spinal cord explants with dorsal root ganglia and murine primary and its role is basically to ensure the skeletal muscle functionality. -
Spinal Muscular Atrophy
Spinal Muscular Atrophy U.S. DEPARTMENT OF HEALTHAND HUMAN SERVICES National Institutes of Health Spinal Muscular Atrophy What is spinal muscular atrophy? pinal muscular atrophy (SMA) is a group Sof hereditary diseases that progressively destroys motor neurons—nerve cells in the brain stem and spinal cord that control essential skeletal muscle activity such as speaking, walking, breathing, and swallowing, leading to muscle weakness and atrophy. Motor neurons control movement in the arms, legs, chest, face, throat, and tongue. When there are disruptions in the signals between motor neurons and muscles, the muscles gradually weaken, begin wasting away and develop twitching (called fasciculations). What causes SMA? he most common form of SMA is caused by Tdefects in both copies of the survival motor neuron 1 gene (SMN1) on chromosome 5q. This gene produces the survival motor neuron (SMN) protein which maintains the health and normal function of motor neurons. Individuals with SMA have insufficient levels of the SMN protein, which leads to loss of motor neurons in the spinal cord, producing weakness and wasting of the skeletal muscles. This weakness is often more severe in the trunk and upper leg and arm muscles than in muscles of the hands and feet. 1 There are many types of spinal muscular atrophy that are caused by changes in the same genes. Less common forms of SMA are caused by mutations in other genes including the VAPB gene located on chromosome 20, the DYNC1H1 gene on chromosome 14, the BICD2 gene on chromosome 9, and the UBA1 gene on the X chromosome. The types differ in age of onset and severity of muscle weakness; however, there is overlap between the types. -

Isolated Brachialis Muscle Atrophy
A Case Report & Literature Review Isolated Brachialis Muscle Atrophy John W. Karl, MD, MPH, Michael T. Krosin, MD, and Robert J. Strauch, MD or sensory complaints. His medical history was otherwise Abstract unremarkable. Physical examination revealed obvious wast- Isolated brachialis muscle atrophy, a rare entity with ing of the right brachialis muscle, most notable on the lateral few reported cases in the literature, is explained by a aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle variety of etiologies. We present a case of unilateral, was functioning with full strength and had a normal bulk. He isolated brachialis muscle atrophy that likely resulted had a normal range of active and passive motion, including from neuralgic amyotrophy. full extension and flexion of both elbows, as well as complete Figure 1. Frontal view of both arms: note the brachialis atrophy solated brachialis muscle atrophy has been rarely reported. (solid arrow) on the right side, although the biceps contracts well. Among the few cases in the literature, 1 was attributed I to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We pres- ent a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report. AJO Case Report A 37-year-old right-handed highway worker presented for eval- uation of right-arm muscle atrophy. One year earlier, while lift- ing heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling.