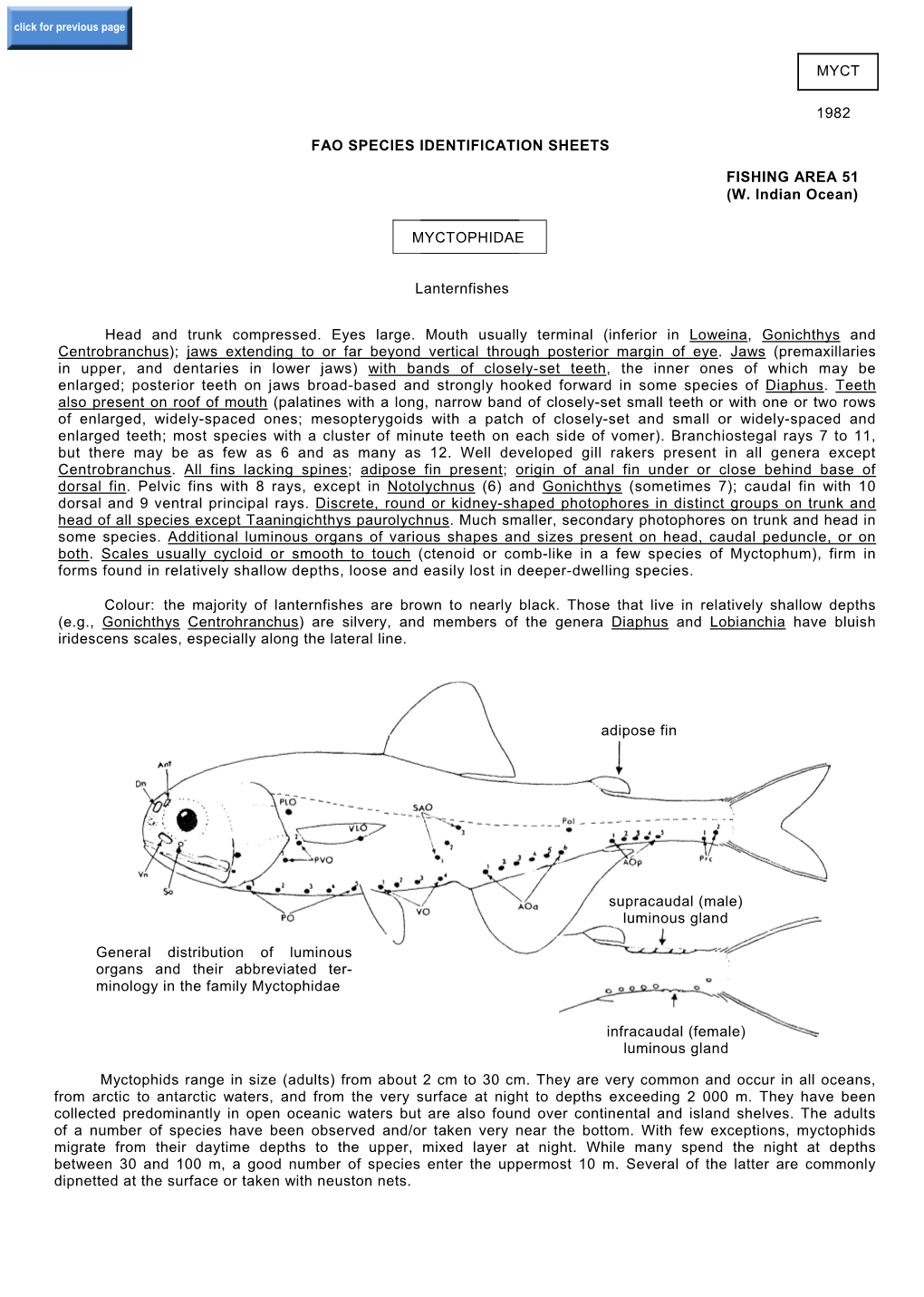
MYCTOPHIDAE Lanternfishes Head and Trunk Compresse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

1 Exon Probe Sets and Bioinformatics Pipelines for All Levels of Fish Phylogenomics
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.18.949735; this version posted February 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Exon probe sets and bioinformatics pipelines for all levels of fish phylogenomics 2 3 Lily C. Hughes1,2,3,*, Guillermo Ortí1,3, Hadeel Saad1, Chenhong Li4, William T. White5, Carole 4 C. Baldwin3, Keith A. Crandall1,2, Dahiana Arcila3,6,7, and Ricardo Betancur-R.7 5 6 1 Department of Biological Sciences, George Washington University, Washington, D.C., U.S.A. 7 2 Computational Biology Institute, Milken Institute of Public Health, George Washington 8 University, Washington, D.C., U.S.A. 9 3 Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian 10 Institution, Washington, D.C., U.S.A. 11 4 College of Fisheries and Life Sciences, Shanghai Ocean University, Shanghai, China 12 5 CSIRO Australian National Fish Collection, National Research Collections of Australia, 13 Hobart, TAS, Australia 14 6 Sam Noble Oklahoma Museum of Natural History, Norman, O.K., U.S.A. 15 7 Department of Biology, University of Oklahoma, Norman, O.K., U.S.A. 16 17 *Corresponding author: Lily C. Hughes, [email protected]. 18 Current address: Department of Organismal Biology and Anatomy, University of Chicago, 19 Chicago, IL. 20 21 Keywords: Actinopterygii, Protein coding, Systematics, Phylogenetics, Evolution, Target 22 capture 23 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.18.949735; this version posted February 19, 2020. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

XIV. Appendices
Appendix 1, Page 1 XIV. Appendices Appendix 1. Vertebrate Species of Alaska1 * Threatened/Endangered Fishes Scientific Name Common Name Eptatretus deani black hagfish Lampetra tridentata Pacific lamprey Lampetra camtschatica Arctic lamprey Lampetra alaskense Alaskan brook lamprey Lampetra ayresii river lamprey Lampetra richardsoni western brook lamprey Hydrolagus colliei spotted ratfish Prionace glauca blue shark Apristurus brunneus brown cat shark Lamna ditropis salmon shark Carcharodon carcharias white shark Cetorhinus maximus basking shark Hexanchus griseus bluntnose sixgill shark Somniosus pacificus Pacific sleeper shark Squalus acanthias spiny dogfish Raja binoculata big skate Raja rhina longnose skate Bathyraja parmifera Alaska skate Bathyraja aleutica Aleutian skate Bathyraja interrupta sandpaper skate Bathyraja lindbergi Commander skate Bathyraja abyssicola deepsea skate Bathyraja maculata whiteblotched skate Bathyraja minispinosa whitebrow skate Bathyraja trachura roughtail skate Bathyraja taranetzi mud skate Bathyraja violacea Okhotsk skate Acipenser medirostris green sturgeon Acipenser transmontanus white sturgeon Polyacanthonotus challengeri longnose tapirfish Synaphobranchus affinis slope cutthroat eel Histiobranchus bathybius deepwater cutthroat eel Avocettina infans blackline snipe eel Nemichthys scolopaceus slender snipe eel Alosa sapidissima American shad Clupea pallasii Pacific herring 1 This appendix lists the vertebrate species of Alaska, but it does not include subspecies, even though some of those are featured in the CWCS. -

Order MYCTOPHIFORMES NEOSCOPELIDAE Horizontal Rows
click for previous page 942 Bony Fishes Order MYCTOPHIFORMES NEOSCOPELIDAE Neoscopelids By K.E. Hartel, Harvard University, Massachusetts, USA and J.E. Craddock, Woods Hole Oceanographic Institution, Massachusetts, USA iagnostic characters: Small fishes, usually 15 to 30 cm as adults. Body elongate with no photophores D(Scopelengys) or with 3 rows of large photophores when viewed from below (Neoscopelus).Eyes variable, small to large. Mouth large, extending to or beyond vertical from posterior margin of eye; tongue with photophores around margin in Neoscopelus. Gill rakers 9 to 16. Dorsal fin single, its origin above or slightly in front of pelvic fin, well in front of anal fins; 11 to 13 soft rays. Dorsal adipose fin over end of anal fin. Anal-fin origin well behind dorsal-fin base, anal fin with 10 to 14 soft rays. Pectoral fins long, reaching to about anus, anal fin with 15 to 19 rays.Pelvic fins large, usually reaching to anus.Scales large, cycloid, and de- ciduous. Colour: reddish silvery in Neoscopelus; blackish in Scopelengys. dorsal adipose fin anal-fin origin well behind dorsal-fin base Habitat, biology, and fisheries: Large adults of Neoscopelus usually benthopelagic below 1 000 m, but subadults mostly in midwater between 500 and 1 000 m in tropical and subtropical areas. Scopelengys meso- to bathypelagic. No known fisheries. Remarks: Three genera and 5 species with Solivomer not known from the Atlantic. All Atlantic species probably circumglobal . Similar families in occurring in area Myctophidae: photophores arranged in groups not in straight horizontal rows (except Taaningichthys paurolychnus which lacks photophores). Anal-fin origin under posterior dorsal-fin anal-fin base. -

Fish Bulletin 161. California Marine Fish Landings for 1972 and Designated Common Names of Certain Marine Organisms of California
UC San Diego Fish Bulletin Title Fish Bulletin 161. California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California Permalink https://escholarship.org/uc/item/93g734v0 Authors Pinkas, Leo Gates, Doyle E Frey, Herbert W Publication Date 1974 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY OF CALIFORNIA DEPARTMENT OF FISH AND GAME FISH BULLETIN 161 California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California By Leo Pinkas Marine Resources Region and By Doyle E. Gates and Herbert W. Frey > Marine Resources Region 1974 1 Figure 1. Geographical areas used to summarize California Fisheries statistics. 2 3 1. CALIFORNIA MARINE FISH LANDINGS FOR 1972 LEO PINKAS Marine Resources Region 1.1. INTRODUCTION The protection, propagation, and wise utilization of California's living marine resources (established as common property by statute, Section 1600, Fish and Game Code) is dependent upon the welding of biological, environment- al, economic, and sociological factors. Fundamental to each of these factors, as well as the entire management pro- cess, are harvest records. The California Department of Fish and Game began gathering commercial fisheries land- ing data in 1916. Commercial fish catches were first published in 1929 for the years 1926 and 1927. This report, the 32nd in the landing series, is for the calendar year 1972. It summarizes commercial fishing activities in marine as well as fresh waters and includes the catches of the sportfishing partyboat fleet. Preliminary landing data are published annually in the circular series which also enumerates certain fishery products produced from the catch. -

Euteleostei: Aulopiformes) and the Timing of Deep-Sea Adaptations ⇑ Matthew P
Molecular Phylogenetics and Evolution 57 (2010) 1194–1208 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Estimating divergence times of lizardfishes and their allies (Euteleostei: Aulopiformes) and the timing of deep-sea adaptations ⇑ Matthew P. Davis a, , Christopher Fielitz b a Museum of Natural Science, Louisiana State University, 119 Foster Hall, Baton Rouge, LA 70803, USA b Department of Biology, Emory & Henry College, Emory, VA 24327, USA article info abstract Article history: The divergence times of lizardfishes (Euteleostei: Aulopiformes) are estimated utilizing a Bayesian Received 18 May 2010 approach in combination with knowledge of the fossil record of teleosts and a taxonomic review of fossil Revised 1 September 2010 aulopiform taxa. These results are integrated with a study of character evolution regarding deep-sea evo- Accepted 7 September 2010 lutionary adaptations in the clade, including simultaneous hermaphroditism and tubular eyes. Diver- Available online 18 September 2010 gence time estimations recover that the stem species of the lizardfishes arose during the Early Cretaceous/Late Jurassic in a marine environment with separate sexes, and laterally directed, round eyes. Keywords: Tubular eyes have arisen independently at different times in three deep-sea pelagic predatory aulopiform Phylogenetics lineages. Simultaneous hermaphroditism evolved a single time in the stem species of the suborder Character evolution Deep-sea Alepisauroidei, the clade of deep-sea aulopiforms during the Early Cretaceous. This result indicates the Euteleostei oldest known evolutionary event of simultaneous hermaphroditism in vertebrates, with the Alepisauroidei Hermaphroditism being the largest vertebrate clade with this reproductive strategy. Divergence times Ó 2010 Elsevier Inc. -

A Review of Lanternfishes (Families: Myctophidae and Neoscopelidae)
Zoological Studies 40(2): 103-126 (2001) A Review of Lanternfishes (Families: Myctophidae and Neoscopelidae) and Their Distributions around Taiwan and the Tungsha Islands with Notes on Seventeen New Records John Ta-Ming Wang* and Che-Tsung Chen Graduate School of Fisheries Science, National Taiwan Ocean University, Keelung, Taiwan 202, R.O.C. Fax: 886-2-28106688. E-mail: [email protected] (Accepted December 20, 2000) John Ta-Ming Wang and Che-Tsung Chen (2001) A review of lanternfishes (Families: Myctophidae and Neoscopelidae) and their distributions around Taiwan and the Tungsha Islands with notes on seventeen new records. Zoological Studies 40(2): 103-126. Lanternfishes collected during 9 cruises from 1991 to 1997 were studied. The area sampled lies between 19°N and 25°N and 114°E and 123°E. The specimens collected in this area comprise 40 species belong to 16 genera, among which 17 species are first records. These first record species include Benthosema fibulatum, Bolinichthys supralateralis, Electrona risso, Hygophum proximum, H. reinhardtii, Lampadena anomala, Lobianchia gemellarii, Lampanyctus niger, L. turneri, L. tenuiformis, Myctophum asperum, M. aurolaternatum, M. nitidulum, M. spinosum, Notolychnus valdiviae, Notoscopelus caudispinosus, and N. resplendens. Among these, six species, Bolinichthys supralateralis, Electrona risso, Lampanyctus turneri, Lampadena anomala, Notolychnus valdiviae, and Notoscopelus caudispinosus, are first records for the South China Sea, and the species, Lampadena anomala is a new record for Asian oceans (Table 1). Four species (Triphoturus microchir, Diaphus diadematus, D. latus, and D. taaningi) were controversial in previous reports, so they are discussed in this study. Geographic distributions and localities of catches of all lanternfish species are shown on the maps (Figs. -
Visual Acuity in Pelagic Fishes and Mollusks
W&M ScholarWorks VIMS Articles 2013 Visual acuity in pelagic fishes and mollusks YL Gagnon TT Sutton S Johnsen Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons Recommended Citation Gagnon, YL; Sutton, TT; and Johnsen, S, "Visual acuity in pelagic fishes and mollusks" (2013). VIMS Articles. 885. https://scholarworks.wm.edu/vimsarticles/885 This Article is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Vision Research 92 (2013) 1–9 Contents lists available at ScienceDirect Vision Research journal homepage: www.elsevier.com/locate/visres Visual acuity in pelagic fishes and mollusks ⇑ Yakir L. Gagnon a, , Tracey T. Sutton b, Sönke Johnsen a a Department of Biology, Duke University, Durham, NC 27708, USA b College of William & Mary, Virginia Institute of Marine Science, Gloucester Point, VA, USA article info abstract Article history: In the sea, visual scenes change dramatically with depth. At shallow and moderate depths (<1000 m), Received 26 June 2013 there is enough light for animals to see the surfaces and shapes of prey, predators, and conspecifics. This Received in revised form 13 August 2013 changes below 1000 m, where no downwelling daylight remains and the only source of light is biolumi- Available online 30 August 2013 nescence. These different visual scenes require different visual adaptations and eye morphologies. In this study we investigate how the optical characteristics of animal lenses correlate with depth and ecology. -

Marine Ecology Progress Series 370:239
Vol. 370: 239–247, 2008 MARINE ECOLOGY PROGRESS SERIES Published October 28 doi: 10.3354/meps07673 Mar Ecol Prog Ser Stable isotopes reveal the trophic position and mesopelagic fish diet of female southern elephant seals breeding on the Kerguelen Islands Y. Cherel1,*, S. Ducatez1, C. Fontaine1, P. Richard2, C. Guinet1 1Centre d’Etudes Biologiques de Chizé, UPR 1934 du CNRS, BP 14, 79360 Villiers-en-Bois, France 2Centre de Recherche sur les Ecosystèmes Littoraux Anthropisés, UMR 6217 du CNRS-IFREMER-ULR, Place du Séminaire, BP 5, 17137 L’Houmeau, France ABSTRACT: Trophic interactions between organisms are the main drivers of ecosystem dynamics, but scant dietary information is available for wide-ranging predators during migration. We investi- gated feeding habits of a key consumer of the Southern Ocean, the southern elephant seal Miroun- gia leonina, by comparing its blood δ13C and δ15N values with those of various marine organisms, including crustaceans, squid, fishes, seabirds and fur seals. At the end of winter, δ13C values (–23.1 to –20.1‰) indicate that female elephant seals forage mainly in the vicinity of the Polar Front and in the Polar Frontal Zone. Trophic levels derived from δ15N values (trophic level = 4.6) show that the southern elephant seal is a top consumer in the pelagic ecosystem that is dominated by colossal squid. The mean δ15N value of seals (10.1 ± 0.3‰) indicates that they are not crustacean eaters, but instead feed on crustacean-eating prey. Surprisingly, most of the previously identified prey species have isotope δ13C and δ15N values that do not fit with those of potential food items. -

Downloaded Tri-Axial Acceleration Data and GPS Files, Analyses
Masello et al. Movement Ecology (2021) 9:24 https://doi.org/10.1186/s40462-021-00255-9 RESEARCH Open Access How animals distribute themselves in space: energy landscapes of Antarctic avian predators Juan F. Masello1* , Andres Barbosa2, Akiko Kato3, Thomas Mattern1,4, Renata Medeiros5,6, Jennifer E. Stockdale5, Marc N. Kümmel7, Paco Bustamante8,9, Josabel Belliure10, Jesús Benzal11, Roger Colominas-Ciuró2, Javier Menéndez-Blázquez2, Sven Griep7, Alexander Goesmann7, William O. C. Symondson5 and Petra Quillfeldt1 Abstract Background: Energy landscapes provide an approach to the mechanistic basis of spatial ecology and decision- making in animals. This is based on the quantification of the variation in the energy costs of movements through a given environment, as well as how these costs vary in time and for different animal populations. Organisms as diverse as fish, mammals, and birds will move in areas of the energy landscape that result in minimised costs and maximised energy gain. Recently, energy landscapes have been used to link energy gain and variable energy costs of foraging to breeding success, revealing their potential use for understanding demographic changes. Methods: Using GPS-temperature-depth and tri-axial accelerometer loggers, stable isotope and molecular analyses of the diet, and leucocyte counts, we studied the response of gentoo (Pygoscelis papua) and chinstrap (Pygoscelis antarcticus) penguins to different energy landscapes and resources. We compared species and gentoo penguin populations with contrasting population trends. Results: Between populations, gentoo penguins from Livingston Island (Antarctica), a site with positive population trends, foraged in energy landscape sectors that implied lower foraging costs per energy gained compared with those around New Island (Falkland/Malvinas Islands; sub-Antarctic), a breeding site with fluctuating energy costs of foraging, breeding success and populations. -

Parvilux, a New Genus of Myctophid Fishes from the Northeastern Pacific, with Two New Species *)
PARVILUX, A NEW GENUS OF MYCTOPHID FISHES FROM THE NORTHEASTERN PACIFIC, WITH TWO NEW SPECIES *) by CARL L. HUBBS and ROBERT L. WISNER University of California, San Diego, U.SA. A relatively gigantic species of lanternfish, described below as represen• ting a new genus, has appeared sparingly since 1950 in bathypelagic collec• tions from off southern California and northern Baja California, Mexico. Although the distinctness of the species has been known for some time, most available specimens had generally been so badly damaged by the nets that an adequate evaluation of the various characters was not possible. Due to a recent increase in collecting effort in these regions, and to the use of a very large midwater trawl, more specimens have been taken than were before available, and a number of these are in a sufficiently good condition to permit the drawing of rather firm conclusions regarding the reliability and constancy of the characters necessary for the indication of the species as sufficiently distinct to warrant the establishment of a new genus. The accumulated study material has been taken on the various cruises of the research vessels of the Scripps Institution of Oceanography and by the Research Vessel "John N. Cobb" during the gear-research and exploratory fishing program of the United States Bureau of Commercial Fisheries. Three additional specimens were taken by the Research Vessel "Velero IV" of the Allan Hancock Foundation of the University of Southern California. In 1952, on the Eastropic Expedition of Scripps Institution, a single specimen of a related species, herein named Parvilux boschmai, was col• lected in a midwater trawl off northern South America, at about 20 N.