Agonimia Opuntiella
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expert Witness Report
Expert Witness Report Report prepared on instructions of: Bleyer Lawyers, Level 1, 550 Lonsdale Street, Melbourne, Vic 3000 Australia Prepared by: Graeme Gillespie B.Sc. Ph.D. 55 Union Street, Northcote, Vic 3070, Australia Curriculum Vitae Attached (Appendix I) I have read the Expert Witness Code of Conduct and agree to be bound by it. Graeme Gillespie 23 February 2010 Qualifications and Experience Please see my curriculum vitae (Appendix I) for my general qualifications and experience. My Ph.D. in zoology focussed specifically on the conservation biology and ecology of frog species in south-eastern Australia. I have 23 years of field and scientific experience studying amphibians and their conservation and management in south- eastern Australia. I have published 24 refereed scientific papers and 38 technical reports on amphibian ecology, conservation and management. I am recognised throughout Australia as an authority on the frog fauna of Victoria, specifically with respect to conservation issues, and I am regularly asked to provide advice on such matters to individuals, government conservation and land management agencies, and non-government organisations. With regard to the Giant Burrowing Frog, I encountered this species on several occasions between 1986 and 1992 while undertaking and supervising pre-logging biodiversity surveys in East Gippsland, Victoria. These records are documented in the Victorian Wildlife Atlas. During this period, I gained knowledge of the species’ habitat associations, breeding biology, some aspects of its behaviour and an appreciation of its conservation status in Victoria (see Opie et al. 1990; Westaway et al.1990; Lobert et al. 1991). Because of my research into amphibian conservation and management, I am highly familiar with the existing literature on the impact of various forest management activities on amphibians and the implications of these activities for amphibian conservation. -

Far East Gippsland Back Road Tours
Far East Gippsland Back Road Tours [Optional Side Trip: Continue travelling 2.5 kms turn right travel 400m Frosty Hollow Campsite] Combienbar Turn north onto Hensleigh Creek Rd travel 9.2 kms East Errinundra Queensborough River Picnic Area. The Queensborough River flows north into the Delegate River which forms part of the upper Snowy River catchment before the 5 river flows westerly and then southerly to Bass Strait. 4WD only. A picturesque route to Continue travelling on Hensleigh Creek Rd 3.9 kms turn eastern Errinundra. left onto Goonmirk Rocks Rd travel 7.6 kms Goonmirk Rocks. A short walk amongst thickets of Mountain Plum-pine. 4WD Classification: Medium Distance: 111 kms continue travelling on Goonmirk Rocks Rd 1.2 kms Duration: Full Day or overnight View from Three Sisters Lookout turn left onto to Gunmark Rd travel 5.2 kms turn left Further Information: onto Errinundra Rd travel 1.9 kms turn left travel 50 m Forests Notes- Tennyson Picnic and Camping Area Errinundra Saddle. This picnic area is the main visitor focus Park Notes- Errinundra National Park for the park. Featuring a high quality interpretative display of the Warnings: Log Truck Traffic Park’s outstanding natural values and an easy 1 km rainforest walk. Seasonal road closures- Goonmirk Rd, Hensleigh Creek Rd, and Tennyson Tk. Continue travelling on Errinundra Rd 4.7 kms Mt Morris Tennyson Tk is steep, rocky and slippery requiring low range Picnic Area. Includes a 2 km 1 hr return walk to the granite 4WD with high ground clearance. A moderately difficult outcrop. Erridundra Plateau is a south east extension of the Monaro route to be travelled with care and by experienced drivers. -

Orbost Spiny Crayfish (Euastacus Diversus)
Action Statement Flora and Fauna Guarantee Act 1988 No. 128 Orbost Spiny Cray Euastacus diversus Description and distribution The Orbost Spiny Crayfish Euastacus diversus (Riek) is a small freshwater crayfish. Members of the genus Euastacus are distinguished by heavy claws or chelae and spiny appearance (Zeidler 1982). The Orbost Spiny Crayfish is distinguished from other Euastacus by the arrangement, number and location of various spines on the exoskeleton. In particular, the species is characterised by the presence of one or two marginal squamal spines, the absence of dorsal thoracic spines, telsonic surface spines or an abdominal boss, the presence of four mesal carpal spines on the claw and a maximum occipital carapace length of 32.2mm (Morgan 1986). The Orbost Spiny Crayfish is olive green in colour (A. Murray pers. obs.) and is one of the smallest species in the genus. Orbost Spiny Crayfish, Euastacus diversus (Approximately life size – © Illustration by Savina The Orbost Spiny Crayfish has one of the most Hopkins) restricted distributions of all Euastacus species, having been found at only seven locations on and around the Errinundra Plateau in East Gippsland. Thirteen individuals were collected from the type locality ‘30 miles north of Orbost’ by Riek in 1956 (Morgan 1986). The species was rediscovered in 1991 when a single specimen was collected in Ferntree Creek, followed in 1994 by records from two sites in Ellery Creek and one site in each of Jungle and Lilly-pilly Creeks, all in the headwaters of the Brodribb River. In 1995 a population was located in Yandown Creek, in the headwaters of the Queensborough River. -
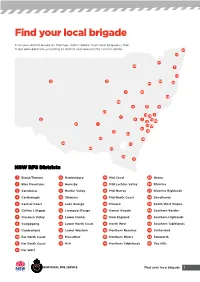
Find Your Local Brigade
Find your local brigade Find your district based on the map and list below. Each local brigade is then listed alphabetically according to district and relevant fire control centre. 10 33 34 29 7 27 12 31 30 44 20 4 18 24 35 8 15 19 25 13 5 3 45 21 6 2 14 9 32 23 1 22 43 41 39 16 42 36 38 26 17 40 37 28 11 NSW RFS Districts 1 Bland/Temora 13 Hawkesbury 24 Mid Coast 35 Orana 2 Blue Mountains 14 Hornsby 25 Mid Lachlan Valley 36 Riverina 3 Canobolas 15 Hunter Valley 26 Mid Murray 37 Riverina Highlands 4 Castlereagh 16 Illawarra 27 Mid North Coast 38 Shoalhaven 5 Central Coast 17 Lake George 28 Monaro 39 South West Slopes 6 Chifley Lithgow 18 Liverpool Range 29 Namoi Gwydir 40 Southern Border 7 Clarence Valley 19 Lower Hunter 30 New England 41 Southern Highlands 8 Cudgegong 20 Lower North Coast 31 North West 42 Southern Tablelands 9 Cumberland 21 Lower Western 32 Northern Beaches 43 Sutherland 10 Far North Coast 22 Macarthur 33 Northern Rivers 44 Tamworth 11 Far South Coast 23 MIA 34 Northern Tablelands 45 The Hills 12 Far West Find your local brigade 1 Find your local brigade 1 Bland/Temora Springdale Kings Plains – Blayney Tara – Bectric Lyndhurst – Blayney Bland FCC Thanowring Mandurama Alleena Millthorpe Back Creek – Bland 2 Blue Mountains Neville Barmedman Blue Mountains FCC Newbridge Bland Creek Bell Panuara – Burnt Yards Blow Clear – Wamboyne Blackheath / Mt Victoria Tallwood Calleen – Girral Blaxland Cabonne FCD Clear Ridge Blue Mtns Group Support Baldry Gubbata Bullaburra Bocobra Kikiora-Anona Faulconbridge Boomey Kildary Glenbrook -

1900 Miscellaneous Land Tenure in Western and Some of Central NSW Mentioned in the 1900 Government Gazette
Rusheen’s Website: www.rusheensweb.com 1900 Miscellaneous Land Tenure in Western and Some of Central NSW mentioned in the 1900 Government Gazette RUSHEEN CRAIG January 2012 Last updated: 4 November 2012 Copyright © 2012 Rusheen Craig Using the information from this document: Please note that the research on this web site is freely provided for personal use only. Site users have the author's permission to utilise this information in personal research, but any use of information and/or data in part or in full for republication in any printed or electronic format (regardless of commercial, non-commercial and/or academic purpose) must be attributed in full to Rusheen Craig. All rights reserved by Rusheen Craig. Miscellaneous Land Tenure Copyright © 2012, Rusheen Craig 1 of 240 Holder Lease Type and Qualification/Location/(Purpose) Area (Acres) Rental or No of Papers Type of Action Number Price (See Legend) £-s-d ABBOTT Louisa After Auction Sold at Corowa.LD & Psh-Mulwala. Co-Denison.Lot 5.Sec 39 …. …. 98-16210 Annulled Purchase a'BECKETT W.C. and a'BECKETT M.E. Preferential No 177; "Nelgowrie"; Central Division. 14,807 …. Occ1900-6564 PrOccL Granted Occupation License aBECKETT William Channing and POccL 177A Central Division. "Nelgowrie". 14,807 115-13-9 …. Pref Occupation aBECKETT Marsham Elwin License. A'BECKETT William Channing and OccL 177 Central Division. "Nelgowrie". 1,010 7-17-10 …. Renewal of Marsham Elwin Occupation License for 1901 ABERNETHY Harold CP 94-1 LD-Wellington.Psh-Guroba.Sec 42. Port 8. 40 …. 98-4768 Certificate of Conformity ABERNETHY Harold CP 95-9 LD-Wellington.Psh-Guroba.Sec 42. -

Destruction of Greater Glider Habitat in Qld and NSW for Development Increased After the Species Was Listed As Vulnerable to Extinction in May 2016
AUSTRALIA - NO / WWF / NO A BRI © Destruction of greater glider habitat in Qld and NSW for development increased after the species was listed as vulnerable to extinction in May 2016. In Victoria habitat where gliders are found is still being clear-cut under an exemption for forestry under national environment law. The 2019/20 summer bushfires scorched nearly a third of the greater glider’s likely habitat. These catastrophic losses highlight several failures of policy and law. Firstly, pervasive failure to enforce Australian environmental laws means that listing the species had no impact in slowing rates of habitat destruction. Rigorous and even handed enforcement is necessary. Second, the exemption for forestry was granted before the greater glider was listed and so fails to take into account the impacts of forestry on the greater glider, allowing their habitats to be logged with little restraint. Such exemptions and special deals for special sectors should be eliminated from the Act. Finally, there is an urgent need for much stronger national and international climate policy to take action to halt climate change, which is driving greater gliders and many other forest dependent wildlife species toward extinction due to the increasing risk of catastrophic bushfires such as those of the 2019-20 summer. Greater gliders (Petauroides volans) are listed as vulnerable nationally under the Environment Protection and Biodiversity Conservation Act (EPBC Act), under the Queensland Nature Conservation Act and also the Victorian Advisory List of Threatened -

Gliding Towards Extinction an Investigation Into Greater Glider Habitat Logged Since the Species Was Listed As Threatened Under the Flora and Fauna Guarantee Act
Gliding towards extinction An investigation into Greater Glider habitat logged since the species was listed as threatened under the Flora and Fauna Guarantee Act Prepared by Goongerah The second anniversary of the Environment Centre, Fauna and listing of the Greater Glider as a Flora Research Collective and threatened species, June 2019. Wildlife of the Central Highlands PHOTO: Josh Bowell An investigation into Greater Glider Goongerah Environment Centre habitat logged since the species was (GECO) is a grassroots community listed as threatened under the Flora and group based in the town of Goongerah Fauna Guarantee Act on 14 June 2017. in far East Gippsland. Since 1993 GECO has campaigned for protection of East Report prepared by Goongerah Gippsland’s high conservation value Environment Centre, Fauna and Flora forests. Research Collective and Wildlife of the Central Highlands. www.geco.org.au Goongerah Environment Centre 7203 Published June 2019 Bonang Rd, Goongerah, 3888 [email protected] Report prepared by Sarah Day [email protected] The Fauna and Flora Research Contributors: Collective Inc. (FFRC) is an independent Dave Caldwell volunteer not-for-profit research Tom Crook collective engaged in surveying for Rena Gaborov threatened species and communities Owen Hanson within Victoria’s public forests that are Ed Hill subject to logging. A Greater Glider found dead inside a logging coupe on the Errinundra Plateau, East Andrew Lincoln Gippsland, in high value Glider habitat. faunaandfloraresearchcollective. wordpress.com Wildlife of the Central Highlands (WOTCH) is an independent volunteer- run grassroots organisation dedicated to protecting Victoria’s native forests through the use of citizen science, community engagement and advocacy. -

Far East Gippsland Back Road Tours
Far East Gippsland Back Road Tours Continue travelling north on the Orbost-Buchan Rd 4.5 kms Stringers (START of Tour 7) turn left onto Monument Tk travel 2.5 kms turn right onto Tower Tk travel 100 m Stringers Knob Knob Historic Fire Tower. This experimental single pole Mottle fire tower was built in 1941 following the disastrous 1939 bushfires. 1 Range The fire spotter's cabin is perched on top of a 28 m tall pole made of 2 massive logs (red iron bark & yellow stringy bark trees) spliced 2WD in dry weather only. lengthwise. Discover a unique monument to a bushman’s ingenuity return to Monument Tk turn right travel 5.3 kms Mottle and impressive flora. Range Flora Reserve. The only known naturally occurring stand of Spotted Gums, Eucalyptus maculata, in Victoria. 2WD 4WD Classification: Easy Spotted Gums, [Optional Side Trip: Long Pt.- Difficult ] Eucalyptus maculata continue travelling on Monument Tk 0.6 kms turn left Distance: 81 kms onto Mottle Range Rd travel 9.2 kms continue south onto Duration: Easy Half Day Wairewa Rd travel 6.5 kms East Gippsland Rail Trail. Further Information: None www.eastgippslandrailtrail.com (at Trestle Bridge). This shared Warnings: Log Truck Traffic. Watch out for cyclists! use path has been developed on the abandoned Bairnsdale/Orbost Optional Side Trip- The road to Long Point has a steep railway corridor for walking, cycling, horse riding. descent, requires a high clearance and is unsuitable for trailers due to a limited turning area. See 4WD classification. Continue travelling on Wairewa Rd through Wairewa 1.3 kms turn left onto the Princes Highway travel 24.3 kms START at Orbost’s Forest Park carpark. -

East Gippsland Area Review
LAND CONSERVATION COUNCIL EAST GIPPSLAND AREA REVIEW FINAL RECOMMENDATIONS December 1986 This text is a facsimile of the former Land Conservation Council’s East Gippsland Area Review Final Recommendations. It has been edited to incorporate Government decisions on the recommendations made by Order in Council dated 15 December 1987 and subsequent formal amendments. Where the Review refers back to the March 1977 East Gippsland Area Final Recommendations, for completeness recommendation wording and Crown descriptions have been reproduced. Added text is shown underlined; deleted text is shown struck through. Annotations [in brackets] explain the origin of changes. 2 MEMBERS OF THE LAND CONSERVATION COUNCIL D. H. F. Scott, B.A. (Chairman) R. W. Campbell, B.Vet.Sc., M.B.Admin.; Director, Land Protection Division (Deputy Chairman) C. N. Austin, C.B.E. D. M. Calder, M.Sc., Ph.D., M.I.Biol. L. Macmillan B.Sc. (Hons) P. A. Eddison, Dip.T.R.P.; Director-General, Department of Conservation, Forests and Lands R. D. Malcolmson, M.B.E., B.Sc., F.A.I.M., M.I.P.M.A., M.Inst.P., M.A.I.P. J. J Wright, B.Sc. (Tech.), M.Eng.Sc., Grad.Dip.O.R.; Chief General Manager, Department of Agriculture and Rural Affairs G. G. Newman, B.Sc., M.Sc., M.B.Admin., Ph.D.; Director, Fisheries Division J. P. Paterson, B.Com., Ph.D.; Director-General, Department of Water Resources D. S. Saunders, B.Agr.Sc., M.A.I.A.S.; Director, National Parks and Wildlife Division R. P. Smith, B.Sc., M.B.Admin., Ph.D.; Director, Public Land Management and Forests Division K. -

Banking Act Unclaimed Money As at 31 December 2007
Commonwealth of Australia Gazette No. ASIC 40A/08, Wednesday, 21 May 2008 Published by ASIC ASIC Gazette Contents Banking Act Unclaimed Money as at 31 December 2007 RIGHTS OF REVIEW Persons affected by certain decisions made by ASIC under the Corporations Act 2001 and the other legislation administered by ASIC may have rights of review. ASIC has published Regulatory Guide 57 Notification of rights of review (RG57) and Information Sheet ASIC decisions – your rights (INFO 9) to assist you to determine whether you have a right of review. You can obtain a copy of these documents from the ASIC Digest, the ASIC website at www.asic.gov.au or from the Administrative Law Co-ordinator in the ASIC office with which you have been dealing. ISSN 1445-6060 (Online version) Available from www.asic.gov.au ISSN 1445-6079 (CD-ROM version) Email [email protected] © Commonwealth of Australia, 2008 This work is copyright. Apart from any use permitted under the Copyright Act 1968, all rights are reserved. Requests for authorisation to reproduce, publish or communicate this work should be made to: Gazette Publisher, Australian Securities and Investment Commission, GPO Box 9827, Melbourne Vic 3001 ASIC GAZETTE Commonwealth of Australia Gazette ASIC 40A/08, Wednesday, 21 May 2008 Banking Act Unclaimed Money Page 2 of 463 Specific disclaimer for Special Gazette relating to Banking Unclaimed Monies The information in this Gazette is provided by Authorised Deposit-taking Institutions to ASIC pursuant to the Banking Act (Commonwealth) 1959. The information is published by ASIC as supplied by the relevant Authorised Deposit-taking Institution and ASIC does not add to the information. -

Government Gazette of the STATE of NEW SOUTH WALES Number 40 Friday, 4 April 2008 Published Under Authority by Government Advertising
2587 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 40 Friday, 4 April 2008 Published under authority by Government Advertising LEGISLATION Regulations New South Wales Motor Vehicles Taxation Amendment (Refunds) Regulation 2008 under the Motor Vehicles Taxation Act 1988 Her Excellency the Governor, with the advice of the Executive Council, has made the following Regulation under the Motor Vehicles Taxation Act 1988. ERIC ROOZENDAAL, M.L.C., MinisterMinister forfor RoadsRoads Explanatory note The object of this Regulation is to amend the Motor Vehicles Taxation Regulation 2003 to recognise that the amount of taxes paid under the Motor Vehicles Taxation Act 1988 that may be refunded on surrender of registration may be calculated on the basis of the number of whole days of registration that are unexpired when a vehicle was stolen or involved in an incident that caused it to be damaged. This Regulation is made under the Motor Vehicles Taxation Act 1988, including sections 13 (b) and 23 (the general regulation-making power). s2007-473-94.d05 Page 1 2588 LEGISLATION 4 April 2008 Clause 1 Motor Vehicles Taxation Amendment (Refunds) Regulation 2008 Motor Vehicles Taxation Amendment (Refunds) Regulation 2008 under the Motor Vehicles Taxation Act 1988 1 Name of Regulation This Regulation is the Motor Vehicles Taxation Amendment (Refunds) Regulation 2008. 2 Amendment of Motor Vehicles Taxation Regulation 2003 The Motor Vehicles Taxation Regulation 2003 is amended as set out in Schedule 1. Page 2 NEW SOUTH WALES GOVERNMENT GAZETTE No. 40 4 April 2008 LEGISLATION 2589 Motor Vehicles Taxation Amendment (Refunds) Regulation 2008 Amendment Schedule 1 Schedule 1 Amendment (Clause 2) Clause 5 Calculation of refund of taxes on surrender of registration Omit the definition of number of days from clause 5 (2). -

Sapphire Coast Is Perfectly Located Midway Between Sydney Merimbula 15 and Melbourne, and Only a Short Bega 16 Drive from Canberra
Sapphirethe far southCoast coast of NSW TOURISM sapphirecoast.com.au Eden | Pambula | Merimbula | Tathra | Bega | Bermagui QUEA THE PILOT Goo Mongarlowe Cunjurong Point Umbango 930m Narra Track Cuumbeun Y M O U N T BA OI N G S O N G R Ck Yaven Creek bar Tallaganda Morton W Lake Conjola Westbrook Ck Tuggeranong NR H (Locality) R NB NP R KINGS (Locality) rag HWY wa Narrawallee Ck NR BATLOW EY boro llee Ck * Yadboro Flat Milton K O S C I U S Z K O and National Ck Downfall Tidbinbilla AN Hoskinstown (Loc) Narrawallee Ck NR Half Moon Flat Yad ra Jerrabomberra (Locality) 719m 3 Kunama NR Bombay Mollymook Carabost Downfall (Locality) Park Mulloon PIGEON HOUSE MTN (Locality) YARROWLUMLA (Locality) Charleys Forest (Locality) R (Locality) Lower Bago * 23 Morton Kings Pt ULLADULLA (Locality) TUMUT (Loc) * SNOWY Braidwood Mongarlowe BUDAWANG Warden Head Tharwa YARROWLUMLA ver Laurel Hill N A T I O N A L P A R K Royalla la Ri Meroo Talbingo Bimberi N A M A D G I THURRALILLY ma NP Rossi River Burrill Lake HILL TALLAGANDA ra g Ck be 4WD only on TALBINGO MTN Burra 982m SHOALHAVEN dd 1394m Burra Dolphin Pt Bu Coolamine NR (Locality) Burra Ck NR Yanununbeyan Farringdon Bendoura Bim 3 HOLBROOK Ck 52 Rosewood (Loc) N A T I O N A L Williamsdale NP (Locality) (Locality) NP R Flat Rock * R Bimberamala (Locality) Mannus Tabourie Lake Cooinbil Jembaicumbene Brooman PRINCESP Courabyra (Locality) Walking C'way Ck NP MTNS (Loc) HARRISONS PEAK (Locality) Carters (Locality) Meroo (Locality) Hardys Hill P A R K 1173m Molonglo Monga * BOURKES HILL (Locality) NP GENBY