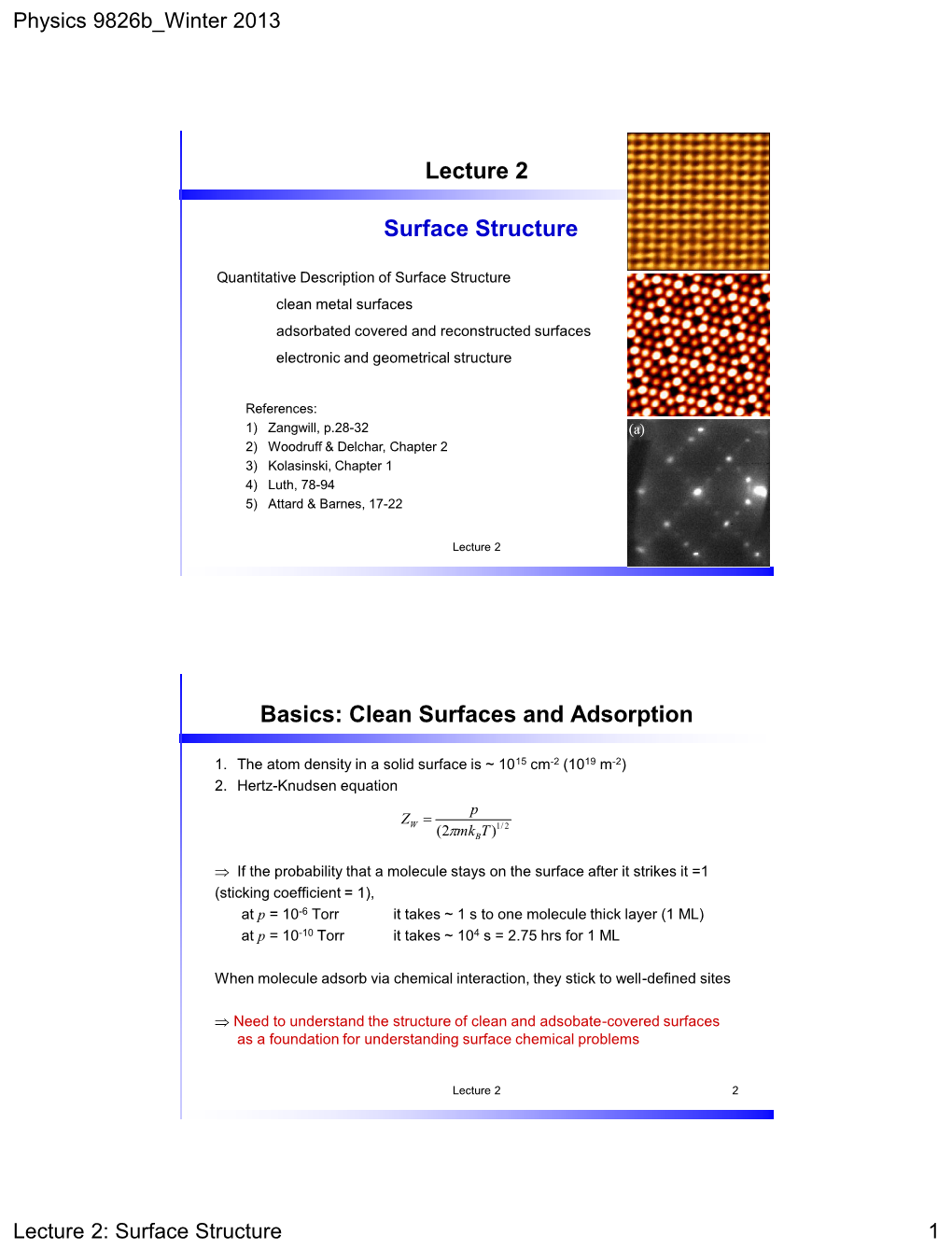
Physics 9826B Winter 2013 Lecture 2: Surface Structure 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chap 1 Crystal Structure
Introduction Example of crystal structure Miller index Chap 1 Crystal structure Dept of Phys M.C. Chang Introduction Example of crystal structure Miller index Bulk structure of matter Crystalline solid Non-crystalline solid Quasi-crystal (1982 ) (amorphous, glass) Ordered, but not periodic 2011 Shechtman www.examplesof.net/2017/08/example-of-solids-crystalline-solids-amorphous-solids.html#.XRHQxuszaJA nmi3.eu/news-and-media/the-contribution-of-neutrons-to-the-study-of-quasicrystals.html Introduction Example of crystal structure Miller index • A simple lattice (in 3D) = a set of points with positions at r = n1a1+n2a2+n3a3 (n1, n2, n3 cover all integers ), a1, a 2, and a3 are called primitive vectors (原始向量), and a1, a 2, and a3 are called lattice constants ( 晶格常數). Alternative definition: • A simple lattice = a set of points in which every point has exactly the same environment. Note: A simple lattice is often called a Bravais lattice . From now on we’ll use the latter term ( not used by Kittel). Introduction Example of crystal structure Miller index Decomposition: Crystal structure = Bravais lattice + basis (of atoms) (How to repeat) (What to repeat) scienceline.ucsb.edu/getkey.php?key=4630 lattice basis 基元 (one atom, or a group of atoms) • Every crystal has a corresponding Bravais lattice and a basis. Note: Lattice is a math term, while crystal is a physics term. 晶格 晶體 Introduction Example of crystal structure Miller index Bravais lattice non-Bravais lattice = Bravais + p-point basis (p>1) Triangular (or hexagonal) lattice Honeycomb lattice Primitive vectors a2 basis a1 Honeycomb lattice = triangular lattice + 2-point basis (i.e. -

Crystal Directions, Wave Propagation and Miller Indices D
Crystal Directions, Wave Propagation and Miller Indices D. K. Ferry, D. Vasileska and G. Klimeck Crystal Directions; Wave Propagation Electron beam generated pattern in TEM (001) (110) Crystal Directions We have referred to various directions in the crystal as (100), (110), and (111). What do these mean? How are these directions determined? Consider a cube- 1 and a plane- 2/3 1/2 The plane has intercepts: x = 0.5a, y = 0.667a, z = a. Crystal Directions We want the NORMAL to the surface. So we take these intercepts (in units of a), and invert them: 1 2 3 , ,1 2, ,1 2 3 2 Then we take the lowest common set of integers: 1 2 3 , ,1 2, ,1 4,3,2 2 3 2 These are the MILLER INDICES of the plane. The NORMAL to the plane is the (4,3,2) direction, which is normally written just (432). (A negative number is indicated by a bar over the top of the number.) Crystal Directions 1 (111) 1 1 (110) 1 1 Crystal Directions (100) 1 (220) 1 1/2 1/2 Crystal Directions Electron beam generated pattern in TEM (001) (110) So far, we have discussed the concept of crystal directions: 1 (111) 1 1 (110) 1 1 We want to say a few more things about this. z Consider this plane. y The intercepts are 0,0, , which leads to 1,1,0 for Miller indices. What are the normals to the plane? x The intercepts are -1, 1, , which leads to 110 z The normal direction is ˆ ˆ x y 110 y x -1 -1 The intercepts are 1, -1, , which leads to 1 10 The normal direction is ˆ ˆ x y 11 0 We can easily shift the planes by one lattice vector in x or y z y x -1 -1 These are two different normals to the same plane. -

Phys 446: Solid State Physics / Optical Properties
Phys 446: Solid State Physics / Optical Properties Fall 2015 Lecture 2 Andrei Sirenko, NJIT 1 Solid State Physics Lecture 2 (Ch. 2.1-2.3, 2.6-2.7) Last week: • Crystals, • Crystal Lattice, • Reciprocal Lattice Today: • Types of bonds in crystals Diffraction from crystals • Importance of the reciprocal lattice concept Lecture 2 Andrei Sirenko, NJIT 2 1 (3) The Hexagonal Closed-packed (HCP) structure Be, Sc, Te, Co, Zn, Y, Zr, Tc, Ru, Gd,Tb, Py, Ho, Er, Tm, Lu, Hf, Re, Os, Tl • The HCP structure is made up of stacking spheres in a ABABAB… configuration • The HCP structure has the primitive cell of the hexagonal lattice, with a basis of two identical atoms • Atom positions: 000, 2/3 1/3 ½ (remember, the unit axes are not all perpendicular) • The number of nearest-neighbours is 12 • The ideal ratio of c/a for Rotated this packing is (8/3)1/2 = 1.633 three times . Lecture 2 Andrei Sirenko, NJITConventional HCP unit 3 cell Crystal Lattice http://www.matter.org.uk/diffraction/geometry/reciprocal_lattice_exercises.htm Lecture 2 Andrei Sirenko, NJIT 4 2 Reciprocal Lattice Lecture 2 Andrei Sirenko, NJIT 5 Some examples of reciprocal lattices 1. Reciprocal lattice to simple cubic lattice 3 a1 = ax, a2 = ay, a3 = az V = a1·(a2a3) = a b1 = (2/a)x, b2 = (2/a)y, b3 = (2/a)z reciprocal lattice is also cubic with lattice constant 2/a 2. Reciprocal lattice to bcc lattice 1 1 a a x y z a2 ax y z 1 2 2 1 1 a ax y z V a a a a3 3 2 1 2 3 2 2 2 2 b y z b x z b x y 1 a 2 a 3 a Lecture 2 Andrei Sirenko, NJIT 6 3 2 got b y z 1 a 2 b x z 2 a 2 b x y 3 a but these are primitive vectors of fcc lattice So, the reciprocal lattice to bcc is fcc. -

Strength of Materials and Damage Assessment - A.V
MECHANICAL ENGINEERING, ENERGY SYSTEMS AND SUSTAINABLE DEVELOPMENT -Vol.1 - Strength of Materials And Damage Assessment - A.V. Berezin STRENGTH OF MATERIALS AND DAMAGE ASSESSMENT A.V. Berezin Laboratory of mathematical modeling of vibroacoustic process, A.A. Blagonravov Institute of Mechanical Engineering, Russian Academy of Sciences, Moscow, Russia Keywords: deformation, stress, strength, fracture, crystal lattice, bond, dislocation, damage. Contents 1. Damage and mechanical behavior of materials 2. General ideas and definitions 3. Structures and bond types of solids 4. A physical view of defects 5. Dislocation mechanisms of origin and growth of cracks 6. Fractography of surfaces of extending cracks 7. Fundamental definitions of defects 8. Methods for evaluating the level of damage Glossary Bibliography Biographical Sketch Summary The physical interpretation of the service life of a material that is under load requires the direct study of evolution of its structure, analysis of connection between elementary phenomena of plastic deformation and damage, and establishing the damage accumulation relationships. The development of increasingly precise methods for studying the microstructure and identifying the defects is associated with the increase of severity of requirements on operation conditions and safety as well as the economic effectiveness of the mechanical structures. Therefore, the problem of determining a change of the damage level in solids becomes more and more urgent in the course of time. UNESCO – EOLSS 1. Damage and Mechanical Behavior of Materials In general, by the level of damage in mechanics we mean a change of the mechanical properties whichSAMPLE may have a different nature CHAPTERS. However, this process is interpreted phenomenologically as the process of forming and growth of microvoids and cracks of various types because of breaking the interatomic bonds. -

Modern Physics Unit 10: Quantum Statistics and Crystalline Solids Lecture 10.2: Unit Cells and Miller Indices
Modern Physics Unit 10: Quantum Statistics and Crystalline Solids Lecture 10.2: Unit Cells and Miller Indices Ron Reifenberger Professor of Physics Purdue University 1 Two Important Concepts 1. A unit cell is the most basic repeating structure of any solid. Atoms in a crystalline solid form a regular repeating pattern. 2. Crystalline solids have periodic arrays of atoms which forms a grid system that fills all space. We call the grid system a crystal lattice. Amorphous solids and glasses are exceptions. 2 Classification of the Unit Cell The unit cell can be classified as primitive or conventional. • There are many ways to define a unit cell •A primitive unit cell has atoms only at the corners of the unit cell. This will be the simplest representation of the crystal structure, but it may not reflect the complete symmetry of the crystal structure. • The conventional unit cell will have atoms at additional sites within the unit cell (i.e. on the faces, at the body center, etc.), causing the conventional unit cell to have the same symmetry as the entire crystal structure. Chosen for convenience. • By international convention, a unit cell is specified to be right- handed, to have the highest symmetry, and to have the smallest cell volume. 3 Visualize crystal structures: http://surfexp.fhi-berlin.mpg.de/SXinput.html Example: There are many possible choices for primitive vectors and primitive unit cells (parallelograms) in two- dimensions non-primitive cell cannot fill all space by translating Area of Primitive cell (in 2D) = a1×= a 2 aa 12sinθ 4 Organizing Space Identify all Specify Unit Cell Rotations, Reflections and Inversions that transform the cell into itself Defines one of 32 possible Four Lattice Types crystallographic Point Groups Primitive Body Centered Face Centered Base Centered Add Space Filling Translations to define Space Groups Find 14 Space Filling Lattices (Bravais Lattices) Bravais Lattices classified into 7 Crystal Systems 7 distinguishable Point Groups 5 The seven crystal systems The 14 Bravais Lattices Lowest symmetry (A. -

Phys 446: Solid State Physics / Optical Properties
Solid State Physics Lecture 2 (Ch. 2.1-2.3, 2.6-2.7) Last week: Phys 446: • Crystals, • Crystal Lattice, Solid State Physics / Optical • Reciprocal Lattice, and Properties • Types of bonds in crystals Today: Fall 2007 • Diffraction from crystals • Importance of the reciprocal lattice concept Lecture 2 Andrei Sirenko, NJIT 1 Lecture 2 Andrei Sirenko, NJIT 2 Crystal Lattice Reciprocal Lattice Lecture 2 Andrei Sirenko, NJIT 3 Lecture 2 Andrei Sirenko, NJIT 4 Diffraction of waves by crystal lattice The Bragg Law • Most methods for determining the atomic structure of crystals are Conditions for a sharp peak in the based on scattering of particles/radiation. intensity of the scattered radiation: • X-rays is one of the types of the radiation which can be used 1) the x-rays should be specularly • Other types include electrons and neutrons reflected by the atoms in one plane • The wavelength of the radiation should be comparable to a typical 2) the reflected rays from the interatomic distance of a few Å (1 Å =10-10 m) successive planes interfere constructively hc hc λ(Å) = 12398/E(eV) ⇒ The path difference between the two x-rays: 2d·sinθ ⇒ E = hν = ⇒ λ = λ E few keV is suitable energy the Bragg formula: 2d·sinθ = mλ for λ = 1 Å The model used to get the Bragg law are greatly oversimplified • X-rays are scattered mostly by electronic shells of atoms in a solid. (but it works!). Nuclei are too heavy to respond. – It says nothing about intensity and width of x-ray diffraction peaks • Reflectivity of x-rays ~10-3-10-5 ⇒ deep penetration into the solid -

Miller Indices and Symmetry Content: a Demonstration Using Shape, a Computer Program for Drawing Crystals
MILLER INDICES AND SYMMETRY CONTENT: A DEMONSTRATION USING SHAPE, A COMPUTER PROGRAM FOR DRAWING CRYSTALS Michael A. Velbel Department of Geological Sciences 206 Natural Science Building Michigan State University East Lansing, MI 48824-1115 [email protected] The purpose of this exercise is to use SHAPE: A COMPUTER PROGRAM FOR DRAWING CRYSTALS to help you visualize the relationship between the morphology of crystals, Miller indices, axial ratios, crystal faces, open and closed forms to the symmetry content of the crystal. Instructions on how to create and modify crystal drawings, and how to customize the display, are included at the end of this exercise; that part of the handout is referred to as the "general instruction" handout. Use it as a reference for the specifics of how to use SHAPE for this lab. These instructions are written for older (MS-DOS) versions of SHAPE. The menu structure in Windows versions is similar. If you can't figure out how to enter some command, ask the instructor for help. In this exercise, you will generate various crystal drawings, copy them from the screen (using colored pencils), and answer questions about intercepts, Miller indices, symmetry, etc., illustrated by each example. The questions you must answer are printed in bold face. Hand in your drawings, and the answers to the questions, at the end of the lab period, keyed to the filename assigned to each drawing below. A. TETRAGONAL (a:c = 1:2) {HI}: First, we will look at how faces with different Miller indices appear in the same crystal class (remember, the crystal class embodies both the coordinate axes and the symmetry content). -

Midterm 01 Solutions
UNIVERSITY OF CALIFORNIA College of Engineering Department of Materials Science & Engineering Professor R. Gronsky Fall Semester 2010 Engineering 45 Midterm 01 Solutions INSTRUCTIONS PATIENCE ................................Do not open pages until “START” is announced. PRUDENCE.............................. Position yourself with occupied seats directly in front of you and vacant seats to your left / right, when possible, unless instructed by exam proctors. PROTOCOL.............................. Only writing instruments / eraser / straightedge are allowed. Remove all other materials, including books / reference materials / calculators / PDAs / cell phones (disable all sounds) / other electronic devices / headphones / ear buds / hats from your person / workplace. POLITENESS ...........................Asking and answering questions during the exam are very disruptive and discourteous to your classmates. So there will be no questions during the exam. Instead, please include your concerns or alternative interpretations in your written answers. PROFESSIONALISM .............The engineering profession demands strict ethical standards of honesty and integrity. Engineers do not cheat on the job, and there will be no cheating on this exam. E45 Fall 10 Midterm 01 Solutions 1. Mechanical Properties The stress-strain plots at right are obtained 533 σt – εt from a single uniaxial tensile test, by varying load (P) and measuring elongation (Δl = l–l0) of a test sample before converting to this 433 σe – εe )*'01$ format. One curve results from a conversion m to engineering stress σe = P/A0 and !"#.//'( 233 engineering strain εe = Δl/l0, the other, true stress σt = P/Ai and true strain εt = Δl/li where the subscripts 0 and i refer to "original" and 3 3632 3634 "instantaneous" values of cross-sectional area !"#$%&'(¡)*'+,-+, (A) and length (l). -

Crystal Structures.Pdf
ELE 362: Structures of Materials James V. Masi, Ph.D. SOLIDS Crystalline solids have of the order of 1028 atoms/cubic meter and have a CRYSTALLINE AMORPHOUS regular arrangement of atoms. Amorphous solids have about the same 1028 atoms/m3 Crystallites approach size density of atoms, but lack the long range of unit cell, i.e. A (10-10 m) order of the crystalline solid (usually no greater than a few Angstroms). Their Regular arrangement crystallites approach the order of the unit cell. The basic building blocks of Basic building blocks are crystalline materials are the unit cells unit cells NO LONG RANGE ORDER which are repeated in space to form the crystal. However, most of the materials Portions perfect-- which we encounter (eg. a copper wire, a polycrystalline-- horseshoe magnet, a sugar cube, a grain boundaries galvanized steel sheet) are what we call polycrystalline. This word implies that portions of the solid are perfect crystals, but not all of these crystals have the same axis of symmetry. Each of the crystals (or grains) have slightly different orientations with boundaries bordering on each other. These borders are called grain boundaries. Crystalline and amorphous solids Polycrystals and grain boundaries Quartz Polycrystal G.B. Movement Crystalline allotropes of carbon (r is the density and Y is the elastic modulus or Young's modulus) Graphite Diamond Buckminsterfullerene Crystal Structure Covalent bonding within Covalently bonded network. Covalently bonded C60 spheroidal layers. Van der Waals Diamond crystal structure. molecules held in an FCC crystal bonding between layers. structure by van der Waals bonding. Hexagonal unit cell. -

X-Ray Fingerprinting Routine for Cut Diamonds
X-RAY FINGERPRINTING ROUTINE FOR CUT DIAMONDS Roland Diehl and Nikolaus Herres X-ray topography is a nondestructive technique that permits the visualization of internal defects in the crystal lattice of a gemstone, especially diamond, which is highly transparent to X-rays. This technique yields a unique “fingerprint” that is not altered by gem cutting or treat- ments such as irradiation and annealing. Although previously a complicated and time-con- suming procedure, this article presents a simplified X-ray topographic routine to fingerprint faceted diamonds. Using the table facet as a point of reference, the sample is crystallographi- cally oriented in a unique and reproducible way in front of the X-ray source so that only one topograph is necessary for fingerprinting. Should the diamond be retrieved after loss or theft, even after recutting or exposure to some forms of treatment, another topograph generated with the same routine could be used to confirm its identity unequivocally. iamonds, whether rough or cut (figure 1), render a faceted diamond uniquely identifiable. are one of the most closely scrutinized com- One method records the pattern of light reflected D modities in the world. Gemological labora- from the facets on a cylindrical sheet of film sur- tories routinely evaluate the carat weight, color, rounding the stone. Such a scintillogram is indeed clarity, and cut characteristics of faceted diamonds, a “fingerprint,” but minor re-polishing will modify and record them on a report that serves not only as subsequent patterns obtained from the same stone a basis for the gem’s value, but also as a record that (Wallner and Vanier, 1992). -

Silicon Basics -- General Overview
Silicon Basics -- General Overview. File: ee4494 silicon basics.ppt revised 09/11/2001 copyright james t yardley 2001 Page 1 Semiconductor Electronics: Review. File: ee4494 silicon basics.ppt revised 09/11/2001 copyright james t yardley 2001 Page 2 Semiconductor Electronics: Review. Atomic Weight 28.09 Silicon: basic Electron configuration [Ne] 3s23p2 Crystal structure Diamond information Lattice constant (Angstrom) 5.43095 and Density: atoms/cm3 4.995E+22 properties. Density (g/cm3) 2.328 Dielectic Constant 11.9 -3 Density of states in conduction band, NC (cm ) 3.22E+19 -3 Density of states in valence band, NV (cm ) 1.83E19 Effective Mass, m*/m0 Electrons m*l 0.98 m*t 0.19 Holes m*l 0.16 m*h 0.49 Electron affinity, x(V) 4.05 Energy gap (eV) at 300K 1.12 File: ee4494 silicon basics.ppt revised 09/11/2001 copyright james t yardley 2001 Page 3 Intrinisic carrier conc. (cm-3) 1.0E10 Intrinsic Debye Length (micron) 24 Silicon: basic Intrinsic resistivity (ohm cm) 2.3 E+05 information Linear coefficient of thermal expansion (1/oC) 2.6 E-06 and Melting point (C) 1415 Minority carrier lifetime (s) 2.5 E-03 properties. 2 Mobility (cm / V sec) m(electrons) 1500 m(holes) 450 Optical-phonon energy (eV) 0.063 Phonon mean free path (Angstrom) 76(electron) 55(hole) Specific heat (J/g oC) 0.7 Thermal conductivity (W/cm oC) 1.5 Thermal diffusivity (cm2/s) 0.9 Vapor pressure (Pa) 1 at 1650C 1E-6 at 900 C Index of refraction 3.42 Breakdown field (V/cm) ~3 E+05 File: ee4494 silicon basics.ppt revised 09/11/2001 copyright james t yardley 2001 Page 4 Crystal structure of silicon (diamond structure). -

Crystal Axes, Systems, Mineral Face Notation (Miller Indices)
Crystal Axes, Systems, Mineral Face Notation (Miller Indices) A CRYSTAL is the outward form of the internal structure of the mineral. The 6 basic crystal systems are: ISOMETRIC HEXAGONAL TETRAGONAL ORTHORHOMBIC Drusy Quartz on Barite MONOCLINIC TRICLINIC Acknowledgement: the following images from Susan and Stan Celestian, Glendale Community College Crystal Systems CRYSTAL SYSTEMS are divided into 6 main groups. The first group is the ISOMETRIC. This literally means “equal measure” and refers to the equal size of the crystal axes. ISOMETRIC - Fluorite Crystals 1 Crystal Systems ISOMETRIC CRYSTALS a3 ISOMETRIC In this crystal system there are 3 axes. Each has the same length (as indicated by a2 the same letter “a”). They all meet at mutual 90o angles in the a1 center of the crystal. Crystals in this system are typically ISOMETRIC blocky or ball-like. Basic Cube ALL ISOMETRIC CRYSTALS HAVE 4 3-FOLD AXES Crystal Systems ISOMETRIC CRYSTALS a3 a3 a1 a2 a2 a1 Fluorite cube with crystal axes. ISOMETRIC - Basic Cube 2 Crystal Systems ISOMETRIC BASIC CRYSTAL SHAPES Spinel Fluorite Pyrite Octahedron Cube Cube with Pyritohedron Striations Garnet Garnet - Dodecahedron Trapezohedron Crystal Systems HEXAGONAL CRYSTALS c HEXAGONAL - Three horizontal axes meeting at angles of 120o and one a3 perpendicular axis. a2 a1 ALL HEXAGONAL CRYSTALS HAVE A SINGLE 3- OR 6-FOLD AXIS = C HEXAGONAL Crystal Axes 3 Crystal Systems HEXAGONAL CRYSTALS These hexagonal CALCITE crystals nicely show the six sided prisms as well as the basal pinacoid. Two subsytems: 1. Hexagonal 2. Trigonal Crystal Systems TETRAGONAL CRYSTALS c TETRAGONAL Two equal, horizontal, mutually perpendicular axes (a1, a2) Vertical axis (c) is perpendicular to the a2 a1 horizontal axes and is of a different length.