A Biomechanical Investigation of Load Sharing at the Distal Forearm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lag Screw Fixation for Treatment of an Ulnar Styloid Process Fracture with Antebrachiocarpal Joint Luxation in a Cat
Published online: 2019-06-04 THIEME e32 Case Report Lag Screw Fixation for Treatment of an Ulnar Styloid Process Fracture with Antebrachiocarpal Joint Luxation in a Cat Lindsay A. Parker1 Amy S. Kapatkin2 Mark C. Fuller3 1 Department of Companion Animals, Atlantic Veterinary College, Address for correspondence Lindsay A. Parker, DVM, Atlantic University of Prince Edward Island, Charlottetown, Canada Veterinary College, University of Prince Edward Island, 550 University 2 Department of Surgical and Radiological Sciences, University of Avenue, Charlottetown, PEI, C1A 4P3, Canada California, Davis, CA, United States (e-mail: [email protected]). 3 Fuller Animal Specialty Surgery, Airdrie, AB, Canada VCOT Open 2019;2:e32–e35. Abstract Fracture of the ulnar styloid process can result in clinically debilitating antebrachio- carpal instability. Previously described repair methods include Kirschner wire and figure Keywords of eight tension band wire and/or intramedullary pinning. This case report describes ► feline ulnar styloid surgical repair of a short oblique ulnar styloid process fracture in a cat using two fracture cortical screws placed in lag fashion. Recheck evaluation confirmed lameness-free full ► osteosynthesis return to function with anatomical fracture healing. Introduction acic limb and, on examination under sedation, was found to Lateral styloid process fractures generally require surgical have a marked soft tissue swelling associated with the distal stabilization to avoid carpal instability and to act as a right antebrachium and increased lateral carpal laxity. physical buttress to provide normal joint function.1 Due to Radiographs revealed an antebrachiocarpal joint luxa- the small diameter of the feline distal ulna, these types of tion with an oblique ulnar styloid process fracture (►Fig. -

Conservative Vs. Surgical Management of Ulnar Styloid Fractures Associated with Distal Radius Fractures
CLINICAL RESEARCH Conservative vs. surgical management of ulnar styloid fractures associated with distal radius fractures Cristian Robles, Santiago Iglesias, Christian Allende Nores, Pablo Rotella, Martín Caloia, Miguel Capomassi Orthopedics and Traumatology Department, Sanatorio Allende (Córdoba, Argentina) ABSTRACT Objectives: To evaluate potential differences in clinical and radiological outcomes after surgical versus conservative management of ulnar styloid fractures associated with unstable distal radius fractures treated by locked volar plating. Materials and Methods: This was a multicenter, retrospective and descriptive study including surgical patients treated at four different institutions between 2009 and 2012 for ulnar styloid fractures associated with unstable distal radius fractures. Ulnar styloid fractures were treated con- servatively in group I and surgically in group II. Results: The average follow-up was 56 months. The study included 57 patients divided into two groups (group I [29 cases] and group II [28 cases]). Patients in group II had 2.76 times (95% CI: 1.086; 8.80) more chances of achieving bone union than those in group I. DASH and pain scores, both at rest and during activity, did not show significant differences between the two groups (p = 0.276 and p = 0.877). Group I presented milder ulnar deviation and better strength (p = 0.0194 and p = 0.024). Conclusions: Although patients who underwent surgery for ulnar styloid fractures had 2.76 more chances of achieving bone union than those who received conservative management, there were no significant differences between both groups in subjective evaluations (DASH and pain scores) or when considering the degree of ulnar styloid involve- ment. However, the parameters of strength and ulnar deviation were better in the conservative management group. -

2.0 Mm LCP Distal Ulna Plate TG
2.0 mm LCP Distal Ulna Plate. For capital and subcapital fractures of the ulna. Technique Guide Table of Contents Introduction 2.0 mm LCP Distal Ulna Plate 2 Indications 4 Surgical Technique Clinical Examples 5 Approach 6 Reduce Fracture and Position Plate 7 Fix Plate Distally 9 Adjust Length and Complete Fixation 11 Closure 13 Implant Removal 13 Product Information Implants 14 Instruments 16 IMPORTANT: This device has not been evaluated for safety and compatibility in the MR environment. This device has not been tested for heating or migration in the MR environment. Image intensifier control Synthes 2.0 mm LCP Distal Ulna Plate. For capital and subcapital fractures of the ulna. The distal ulna is an essential component of the distal radioulnar joint, which helps provide rotation to the forearm. The distal ulnar surface is also an important platform for stability of the carpus and, beyond it, the hand. Unstable fractures of the distal ulna therefore threaten both movement and stability of the wrist. The size and shape of the distal ulna, combined with the overlying mobile soft tissues, make application of standard implants difficult. The 2.0 mm LCP Distal Ulna Plate is specifically designed for use in fractures of the distal ulna. Features – Pointed hooks and locking screws in the head – Anatomically precontoured – Angular stability 2 Synthes 2.0 mm LCP Distal Ulna Plate Technique Guide Narrow plate design, low screw-plate profile, rounded edges and polished surface are designed to minimize irritation of overlying soft tissue Pointed hooks -

Effect of Ulnar Styloid Fracture on Functional Outcome of Colle's Fractures
International Surgery Journal Dar IH et al. Int Surg J. 2015 Nov;2(4):556-559 http://www.ijsurgery.com pISSN 2349-3305 | eISSN 2349-2902 DOI: http://dx.doi.org/10.18203/2349-2902.isj20151079 Research Article Effect of ulnar styloid fracture on functional outcome of Colle’s fractures: a comparative analysis of two groups Imtiyaz Hussain Dar1, Iftikhar H. Wani1*, Ummar Mumtaz1, Masrat Jan2 1Department of Orthopedics, Government Medical College Hospital for Bone and Joint Surgery, Srinagar-190005, Kashmir, India 2 Department of Anesthesia and Intensive Care, Government Medical College, Srinagar-190005, Kashmir, India Received: 21 July 2015 Accepted: 24 August 2015 *Correspondence: Dr. Iftikhar H. Wani, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: The negative impact of ulnar sided injuries on distal radius fractures has opened another field of research and is gaining more attention. The aim of our study is to assess the impact of ulnar styloid process fracture on functional outcome of distal radius fractures managed conservatively. Methods: Radiological and Medical records of 150 patients of distal radius with and without styloid process fractures were retrospectively reviewed. Fractures were classified as per Frykman’s classification and patients were assessed for pain, grip and range of motion in addition to other parameters in Mayo’s wrist score. The instability at distal radioulnar joint was evaluated and compared in two groups. -

Surgical Management of Ulnar Styloid Fractures
Chen et al. Journal of Orthopaedic Surgery and Research (2020) 15:273 https://doi.org/10.1186/s13018-020-01795-3 RESEARCH ARTICLE Open Access Surgical management of ulnar styloid fractures: comparison of fixation with anchor suture and tension band wire Alvin Chao-Yu Chen* , Yi-Hsuan Lin, Chun-Jui Weng and Chun-Ying Cheng Abstract Background: Limited reference is available regarding surgical management in symptomatic ulnar styloid fractures with small bony avulsion. The study goal is to report the surgical outcomes using anchor suture fixation with comparison to traditional tension band wire fixation. Methods: We retrospectively reviewed the medical records in patients who underwent surgical repair for unilateral ulnar styloid fractures with distal radioulnar instability between 2004 and 2017. A total of 31 patients were enrolled including two kinds of fixation methods. Anchor suture fixation plus distal radioulnar joint pinning was performed in ten patients with tiny avulsion bony fragments (group A); tension band wire fixation was performed in 21 patients with big styloid fracture fragments (group B). Patient characteristics and 2-year treatment outcomes were compared between two groups based on Mayo Modified Wrist Score (MMWS); Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH); visual analog scale (VAS), and surgical complication. Descriptive statistics were used for calculation of key variables; a p value of < 0.05 was considered statistically significant. Results: Based on Gaulke classification, there were five subtypes in group A and three subtypes in group B. Incidence of concomitant distal radius fractures was significantly higher in group B; other patient characteristics including age, sex, injury side, and time to surgery showed no significant difference. -

ARMASSIST Instruction Video
Scan the QR code to view a Measuring ARMASSIST Instruction video P.O. # Account # Contact Date Phone Patient Name Age Height Weight Company Name Dropship Name & Address PRODUCT INFORMATION Arm Foam ■ LEFT ■ RIGHT ■ FLAT ■ WAVEFOAM A PDF of this order form can be found online at bit.ly/SIGmccCustom CIRCUMFERENCE MEASUREMENTS ANTERIOR AXILLA POSTERIOR AXILLA 25 cm 20 cm A B LENGTH LENGTH 15 cm 10 cm 5 cm OLECRANON PROCESS ELBOW CREASE ELBOW 5 cm 10 cm 15 cm = Locations measured C along Dorsal aspect LENGTH 20 cm 25 cm ULNAR STYLOID D LENGTH Third metacarpal head E E WIDTH Width of hand across dorsal Metacarpal Heads [email protected] • P: 800-322-7744 • F: 800-481-5488 ARMASSIST MEASURING INSTRUCTIONS IMPORTANT Measuring for this garment requires special measurements critical to proper fit. Before you begin, attend live training at a SIGVARIS Certified Compression Specialist course near you (info at sigvariseducation.com). Alternatively, call Customer Care at 800-322-7744, or e-mail [email protected], to receive a remote consultation/training. SUPPLIES NEEDED 1. Cell phone with camera. Photos of the arm(s) with measurement markings MUST be e-mailed to: [email protected]. 2. Measuring Instructions and Forms. 3. SIGVARIS Measuring tape and Body Pen (or eyeliner pencil). 4. Signed Custom Order Terms & Conditions Form (include with order). INSTRUCTIONS All measurements should be recorded in centimeters. Apply slight tension to hold the tape measure in place. STEP 1: LOCATE LANDMARKS 1. Encircle arm with tape measure located as high as possible at Axilla. Mark on the proximal edge of tape at the Anterior, Dorsal, and Posterior aspects of arm, and record the circumference on the line labeled Posterior Axilla. -

Surgical Approaches to the Distal Radioulnar Joint Gregory I
Techniques in Hand and Upper Extremity Surgery 11(1):51–56, 2007 Ó 2007 Lippincott Williams & Wilkins, Philadelphia | DISTAL RADIOULNAR JOINT SYMPOSIUM | Surgical Approaches to the Distal Radioulnar Joint Gregory I. Bain, MBBS, FRACS and Nicholas Pourgiezis, MBBS Modbury Public Hospital, Modbury and University of Adelaide North Terrace, Adelaide South Australia, Australia James H. Roth, MD, FRCSC, FRCS Hand and Upper Limb Centre St Joseph’s Health Centre Division of Orthopaedic Surgery Department of Surgery and the University of Western Ontario London, Ontario, Canada | ABSTRACT retinaculum and used as an interposition flap stabilizing 2 The distal radioulnar joint (DRUJ) is classified as a the DRUJ. In a simplified technique, a composite interposition flap comprising extensor retinaculum and uniaxial synovial pivot joint between the convex head of dorsal capsule is used for reconstruction. This technique the ulna and the concave ulnar notch of the radius. was first described by Stanley and Herbert3 for The DRUJ can be approached from 3 sides with a reconstruction of the DRUJ with a Swanson ulnar head refined dorsal approach retaining a robust retinacular- spacer and subsequently reported by Bain et al2 for dorsal capsular layer preferred by most surgeons. matched hemiresection interposition arthroplasty of the Recent descriptions of safe and extensile volar 4 approaches have broadened indications for a volar DRUJ and van Schoonhoven and Herbert for use in approach to the DRUJ. A subcutaneous ulnar approach conjunction with an ulnar head prosthesis. Exposure of the volar aspect of the distal forearm is remains an option particularly when dealing with most commonly required for the management of distal additional distal ulnar pathology. -

The Ulna Humerus and Ulna: Landmarks and Articulations
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. The Ulna o the ulna is a medial long bone, the longer of the two in the forearm. Proximally, it articulates with the capitulum and trochlea of the humerus; . At the radial notch, it articulates with the head of radius o It stabilizes the forearm o The HEAD LIES DISTALLY. The Olecranon The Trochlear Notch The Coronoid Process The Radial Notch where the head of the radius goes The Tuberosity of the Ulna is where the Brachialis attaches The deep part of Supinator fossa the supinator Supinator crest attaches here Bony features include: - Olecranon - Trochlear notch - Coronoid process - Radial notch - Tuberosity of ulna - Supinator fosa - Supinator crest - Head of ulna - Ulnar styloid process The head of ulna The ulnar styloid process Humerus and ulna: landmarks and articulations o the humerus is a long bone, the largest in the upper limb o it articulates proximally with the scapula at the scapulohumeral (glenohumeral) joint o it articulates distally with the ulna at the elbow joint o the ulna and humerus articulate at the elbow joint o the articulations include: . articulation between the trochlea of the humerus and the trochlear notch of the ulna . articulation of the olecranon process and the olecranon fossa during extension . articulation of the coronoid process and the coronoid fossa during flexion o the surface landmarks include . the medial and lateral epicondyle . the olecranon . the posterior border of the ulna . -
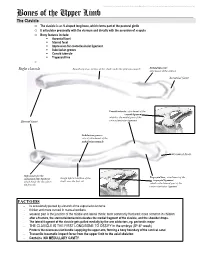
Bones of the Upper Limb
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. Bones of the Upper Limb The Clavicle o The clavicle is an S-shaped long bone, which forms part of the pectoral girdle o It articulates proximally with the sternum and distally with the acromion of scapula o Bony features include: . Acromial facet . Sternal facet . Impression for costoclavicular ligament . Subclavian groove . Conoid tubercle . Trapezoid line o Right clavicle Smooth superior surface of the shaft, under the platysma muscle Deltoid tubercle: attachment of the deltoid Acromial facet Conoid tubercle, attachment of the conoid ligament which is the medial part of the Sternal facet coracoclavicular ligament Subclavian groove: site of attachment of the subclavius muscle Acromial facet Impression for the Trapezoid line, attachment of the costoclavicular ligament Rough inferior surface of the trapezoid ligament which binds the clavicle to shaft, over the first rib which is the lateral part of the the first rib coracoclavicular ligament FACTOIDS - Its occasionally pierced by a branch of the supraclavicular nerve - thicker and more curved in manual workers - weakest part is the junction of the middle and lateral thirds: most commonly fractured; more common in children - after a fracture, the sternocleidomastoid elevates the medial fragment of the clavicle, and the shoulder drops. - The lateral fragment of the clavicle gets pulled medially by the arm adductors, eg. pectoralis major - THE CLAVICLE IS THE FIRST LONG BONE TO OSSIFY in the embryo (5th-6th week) - Protects the neurovascular bundle supplying the upper arm, forming a bony boundary of the cervical canal - Transmits traumatic impact force from the upper limb to the axial skeleton - Contains NO MEDULLARY CAVITY - This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. -

Early and Late Fixation of Ulnar Styloid Base Fractures Yields Different
Chen et al. Journal of Orthopaedic Surgery and Research (2018) 13:193 https://doi.org/10.1186/s13018-018-0899-6 RESEARCH ARTICLE Open Access Early and late fixation of ulnar styloid base fractures yields different outcomes Alvin Chao-Yu Chen* , Chih-Hao Chiu, Chun-Jui Weng, Shih-Sheng Chang and Chun-Ying Cheng Abstract Background: The role of surgical fixation of ulnar styloid fractures remains a subject of debate. The purpose of this study was to compare the surgical outcomes following early and late intervention. Methods: We retrospectively reviewed 28 patients who underwent surgical repair for unilateral ulnar styloid fractures with distal radioulnar instability between 2004 and 2014. Surgical fixation was performed within 3 months of injury in 13 patients (group A) and beyond 3 months in 15 patients (group B). Patient characteristics and functional outcomes were compared between the two groups. The outcome survey consisted of QuickDASH score, grip strength, range of motion, pain score based on the visual analog scale, and surgical complications. Descriptive statistics were calculated for key variables. A p value of < 0.01 was considered statistically significant. Results: Patient characteristics including age, sex, injured side, dominant side injury, and concomitant distal radius fracture showed no significant differences between the two groups. Time to surgery averaged 1.1 months in group A and 12.3 months in group B. Significantly better outcomes were found in group A than in group B, including QuickDASH scores (4.4 ± 5.9 vs. 12.9 ± 9.9) and grip strength (37.4 ± 5.1 vs. 29.1 ± 5.9 kg). -

Anatomy Lab: the Skeletal System Part I: Vertebrae and Thoracic Cage
ANA Lab: Bone 1 Anatomy Lab: The skeletal system Part I: Vertebrae and Thoracic cage Spine (Vertebrae) Body Vertebral arch Vertebral canal Pedicle Lamina Spinous process Transverse process Sup. articular facets Inf. articular facets Sup. vertebral notch Inf. vertebral notch Intervertebral foramen Cervical vertebrae: 7 Typical (C3-C6) Transverse foramen C1, Atlas C2, Axis: dens C7 Thoracic vertebrae: 12 Typical (T2-T10) T1 T11, 12 Lumbar vertebrae: 5 Typical (L1-4) Sacrum: 5 Ala Anterior sacral foramina Posterior sacral foramina Sacral canal ANA Lab: Bone 2 Sacral hiatus promontory median sacral crest intermediate crest lateral crest Coccyx Horns Transverse process Thoracic cages Ribs: 12 pairs Typical ribs (R3-R10): Head, 2 facets intermediate crest neck tubercle angle costal cartilage costal groove R1 R2 R11,12 Sternum Manubrium of sternum Clavicular notch for sternoclavicular joint body xiphoid process ANA Lab: Bone 3 Part II: Skull and Facial skeleton Skull Cranial skeleton, Calvaria (neurocranium) Facial skeleton (viscerocranium) Overview: identify the margin of each bone Cranial skeleton 1. Lateral view Frontal Temporal Parietal Occipital 2. Cranial base midline: Ethmoid, Sphenoid, Occipital bilateral: Temporal Viscerocranium 1. Anterior view Ethmoid, Vomer, Mandible Maxilla, Zygoma, Nasal, Lacrimal, Inferior nasal chonae, Palatine 2. Inferior view Palatine, Maxilla, Zygoma Sutures: external view vs. internal view Coronal suture Sagittal suture Lambdoid suture External appearance of skull Posterior view external occipital protuberance -

Dorsoulnar Wrist Ganglion Associated with Os Ulnostyloideum
A Case Report with Literature Review Dorsoulnar Wrist Ganglion Associated With The Role of Os Ulnostyloideum: A Case Report Aspirin Obi U. Osuji, MD, and Timothy R. McAdams, MD Keith R rue accessory ossifications of the ulnar wrist, unrelated to prior trauma, are uncommon.1 In a study of 800 asymptomatic wrists, Biyani and colleagues2 found only 2 cases of an accessory Tos styloideum, or “os ulnostyloideum.” Accessory ossicles in the immediate area surrounding the distal ulna and its styloid process have been implicated in primary chronic polyarthritis and inflammation.1,3 Wrist ganglions are typically found on the dorsal or volar aspect of the wrist. Ulnar-sided dorsal ganglions are less common and, when present, usually arise from the scapholunate joint and spread ulnarly. To our knowledge, ulnar-sided ganglion cysts have not been described in rela- tion to accessory ossifications of the ulnar styloid. Figure 1. Preoperative clinical photograph of the dorsal wrist shows an ulnar-sided mass about the ulnar styloid. CASE REPORT A right-hand–dominant woman in her early 30s presented gelatinous contents consistent with a ganglion were visual- with a mass on the dorsoulnar aspect of the left wrist ized within the mass. The stalk was transected at its base, and complained of occasional pain on contact (Figure 1). and the mass was sent for pathologic review. Patient history was unremarkable except for a hypothyroid Next, a 1x1x0.5-cm ossicle was found distal to the ulnar condition controlled with levothyroxine. There was no styloid (Figure 3). Subperiosteal elevation allowed excision remote history of trauma to the wrist.