Australian Tropical Rainforest Plants - Online Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Pouteria Sapota, Sapotaceae) from Southeastern Mexico: Its Putative Domestication Center
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 7-6-2019 Structure and genetic diversity in wild and cultivated populations of Zapote mamey (Pouteria sapota, Sapotaceae) from southeastern Mexico: its putative domestication center Jaime Martínez-Castillo Centro de Investigación Científica de ucatánY (CICY), [email protected] Nassib H. Blancarte-Jasso Centro de Investigación Científica de ucatánY (CICY) Gabriel Chepe-Cruz Centro de Investigación Científica de ucatánY (CICY) Noemí G. Nah-Chan Centro de Investigación Científica de ucatánY (CICY) Matilde M. Ortiz-García Centro de Investigación Científica de ucatánY (CICY) See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Martínez-Castillo, Jaime; Blancarte-Jasso, Nassib H.; Chepe-Cruz, Gabriel; Nah-Chan, Noemí G.; Ortiz- García, Matilde M.; and Arias, Renee S., "Structure and genetic diversity in wild and cultivated populations of Zapote mamey (Pouteria sapota, Sapotaceae) from southeastern Mexico: its putative domestication center" (2019). Publications from USDA-ARS / UNL Faculty. 2200. https://digitalcommons.unl.edu/usdaarsfacpub/2200 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Jaime Martínez-Castillo, Nassib H. -

Renata Gabriela Vila Nova De Lima Filogenia E Distribuição
RENATA GABRIELA VILA NOVA DE LIMA FILOGENIA E DISTRIBUIÇÃO GEOGRÁFICA DE CHRYSOPHYLLUM L. COM ÊNFASE NA SEÇÃO VILLOCUSPIS A. DC. (SAPOTACEAE) RECIFE 2019 RENATA GABRIELA VILA NOVA DE LIMA FILOGENIA E DISTRIBUIÇÃO GEOGRÁFICA DE CHRYSOPHYLLUM L. COM ÊNFASE NA SEÇÃO VILLOCUSPIS A. DC. (SAPOTACEAE) Dissertação apresentada ao Programa de Pós-graduação em Botânica da Universidade Federal Rural de Pernambuco (UFRPE), como requisito para a obtenção do título de Mestre em Botânica. Orientadora: Carmen Silvia Zickel Coorientador: André Olmos Simões Coorientadora: Liliane Ferreira Lima RECIFE 2019 Dados Internacionais de Catalogação na Publicação (CIP) Sistema Integrado de Bibliotecas da UFRPE Biblioteca Central, Recife-PE, Brasil L732f Lima, Renata Gabriela Vila Nova de Filogenia e distribuição geográfica de Chrysophyllum L. com ênfase na seção Villocuspis A. DC. (Sapotaceae) / Renata Gabriela Vila Nova de Lima. – 2019. 98 f. : il. Orientadora: Carmen Silvia Zickel. Coorientadores: André Olmos Simões e Liliane Ferreira Lima. Dissertação (Mestrado) – Universidade Federal Rural de Pernambuco, Programa de Pós-Graduação em Botânica, Recife, BR-PE, 2019. Inclui referências e anexo(s). 1. Mata Atlântica 2. Filogenia 3. Plantas florestais 4. Sapotaceae I. Zickel, Carmen Silvia, orient. II. Simões, André Olmos, coorient. III. Lima, Liliane Ferreira, coorient. IV. Título CDD 581 ii RENATA GABRIELA VILA NOVA DE LIMA Filogenia e distribuição geográfica de Chrysophyllum L. com ênfase na seção Villocuspis A. DC. (Sapotaceae Juss.) Dissertação apresentada e -

Pouteria Franciscana Baehni (Chrysophylloideae, Sapotaceae) in Amapá State, Eastern Brazilian Amazonia
16 1 NOTES ON GEOGRAPHIC DISTRIBUTION Check List 16 (1): 27–35 https://doi.org/10.15560/16.1.27 First record of Pouteria franciscana Baehni (Chrysophylloideae, Sapotaceae) in Amapá state, eastern Brazilian Amazonia Caroline da Cruz Vasconcelos1, Mário Henrique Terra-Araujo1, Ana Cláudia Lira-Guedes2, Marcelino Carneiro Guedes2, Janaina Barbosa Pedrosa Costa2 1 Instituto Nacional de Pesquisas da Amazônia (INPA), Programa de Pós-Graduação em Botânica (PPG-BOT), Av. André Araújo 2936, Manaus, Amazonas, 69067-375, Brazil. 2 Empresa Brasileira de Pesquisa Agropecuária (Embrapa Amapá), Núcleo de Recursos Florestais, Rod. Juscelino Kubitschek Km 5 2600, Macapá, Amapá, 68903-419, Brazil. Corresponding author: Caroline da Cruz Vasconcelos, [email protected] Abstract This is the first record of Pouteria franciscana Baehni (Chrysophylloideae, Sapotaceae) in Amapá state, Brazil. We provide a brief description as well as a distribution map, illustrations, and a table with useful features to distinguish P. franciscana from its morphologically related Amazonian species. Using geographic data and applying IUCN criteria, we assign the status as Least Concern for P. franciscana. Keywords “Abiurana”, Amazonian floodplain forest, flora, new occurrence, taxonomy. Academic editor: Adriano Stinca | Received 14 July 2019 | Accepted 20 December 2019 | Published 10 January 2020 Citation: Vasconcelos CC, Terra-Araujo MH, Lira-Guedes AC, Guedes MC, Costa JBP (2020) First record of Pouteria franciscana Baehni (Chrysophylloideae, Sapotaceae) in Amapá state, eastern Brazilian Amazonia. Check List 16 (1): 27–35. https://doi.org/10.15560/16.1.27 Introduction the Cerrado, Caatinga, Amazonia, and Atlantic Forest biomes; the last two are considered the major centers Sapotaceae Juss. is a pantropical woody family divided of diversity for some genera of Sapotaceae (Pennington into three subfamilies: Chrysophylloideae Luerss., Sapo- 1990, 2006; Terra-Araujo et al. -

Rare Plants of Louisiana
Rare Plants of Louisiana Agalinis filicaulis - purple false-foxglove Figwort Family (Scrophulariaceae) Rarity Rank: S2/G3G4 Range: AL, FL, LA, MS Recognition: Photo by John Hays • Short annual, 10 to 50 cm tall, with stems finely wiry, spindly • Stems simple to few-branched • Leaves opposite, scale-like, about 1mm long, barely perceptible to the unaided eye • Flowers few in number, mostly born singly or in pairs from the highest node of a branchlet • Pedicels filiform, 5 to 10 mm long, subtending bracts minute • Calyx 2 mm long, lobes short-deltoid, with broad shallow sinuses between lobes • Corolla lavender-pink, without lines or spots within, 10 to 13 mm long, exterior glabrous • Capsule globe-like, nearly half exerted from calyx Flowering Time: September to November Light Requirement: Full sun to partial shade Wetland Indicator Status: FAC – similar likelihood of occurring in both wetlands and non-wetlands Habitat: Wet longleaf pine flatwoods savannahs and hillside seepage bogs. Threats: • Conversion of habitat to pine plantations (bedding, dense tree spacing, etc.) • Residential and commercial development • Fire exclusion, allowing invasion of habitat by woody species • Hydrologic alteration directly (e.g. ditching) and indirectly (fire suppression allowing higher tree density and more large-diameter trees) Beneficial Management Practices: • Thinning (during very dry periods), targeting off-site species such as loblolly and slash pines for removal • Prescribed burning, establishing a regime consisting of mostly growing season (May-June) burns Rare Plants of Louisiana LA River Basins: Pearl, Pontchartrain, Mermentau, Calcasieu, Sabine Side view of flower. Photo by John Hays References: Godfrey, R. K. and J. W. Wooten. -
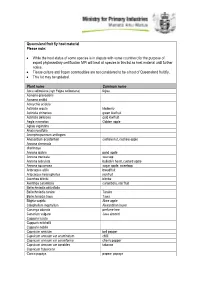
MPI Queensland Fruit Fly Host Material List
Queensland fruit fly host material Please note: While the host status of some species is in dispute with some countries; for the purpose of export phytosanitary certification MPI will treat all species in this list as host material until further notice. Tissue culture and frozen commodities are not considered to be a host of Queensland fruit fly. This list may be updated. Plant name Common name Acca sellowiana (syn Feijoa sellowiana) feijoa Acmena graveolens Acmena smithii Acroychia acidula Actinidia arguta kiwiberry Actinidia chinensis green kiwifruit Actinidia deliciosa gold kiwifruit Aegle marmelos Golden apple Aglaia sapindina Alyxia ruscifolia Amorphospermum antilogum Anacardium occidentale cashew nut, cashew apple Annona cherimola cherimoya Annona glabra pond apple Annona muricata soursop Annona reticulata bullock's heart, custard apple Annona squamosa sugar apple, sweetsop Artocarpus altilis breadfruit Artocarpus heterophyllus jackfruit Averrhoa bilimbi blimbe Averrhoa carambola carambola, star fruit Beilschmiedia obtusifolia Beilschmiedia taraire Taraire Beilschmiedia tawa Tawa Blighia sapida Akee apple Calophyllum inophyllum Alexandrian laurel Cananga odorata perfume tree Canarium vulgare Java almond Capparis lucida Capparis mitchellii Capparis nobilis Capsicum annuum bell pepper Capsicum annuum var acuminatum chilli Capsicum annuum var cerasiforme cherry pepper Capsicum annuum var conoides tabasco Capsicum frutescens Carica papaya papaw, papaya Carica pentagona babaco Carissa ovata Casimiroa edulis white sapote Cassine australia Castanospora alphandii Chrysophyllum cainito caimito, star apple Cissus sp Citrullus lanatus Watermelon Citrus spp. Citrus aurantiifolia West indian lime Citrus aurantium Seville orange Citrus grandis (syn maxima) pummelo Citrus hystrix kaffir lime Citrus jambhiri rough lemon Citrus latifolia Tahatian lime Citrus limetta sweet lemon tree Citrus limon lemon Citrus maxima (syn grandis) pomelo, shaddock Citrus medica citron Citrus meyeri Meyer lemon Citrus reticulata mandarin Citrus reticulata var. -

CIRCULAR Issue No
FDACS-P-01915 CIRCULAR Issue No. 40 | October 2018 Florida Department of Agriculture and Consumer Services Division of Plant Industry The Buckthorns (Genus Sideroxylon): An Underappreciated Group of Florida Native Plants Paul T. Corogin; Bureau of Entomology, Nematology and Plant Pathology [email protected] or 1-888-397-1517 INTRODUCTION Tucked away amongst the rich diversity of Florida plant life surrounding us, one plant group can easily escape our notice: the genus Sideroxylon, belonging to the pantropical family Sapotaceae (sapodilla family). This circular will introduce the Sideroxylon species native to North America, featuring in detail species adapted to the temperate zone that may be of interest to the southern United States (U.S.). Some are endangered in Florida, and some are Florida endemics. Certain species have landscaping potential, but have long been ignored, but a few species are occasionally available from native plant nurseries (Betrock’s Plant Search 2018; FNPS 2018). Species of Sideroxylon attract pollinators when blooming, and birds and wildlife when fruiting; thus, they can be desirable additions to any Florida landscape. Sapotaceae are recognized by the presence of milky sap, brownish T-shaped hairs, fasciculate inflorescences (flowers in a bundle) and seeds with a large scar at one end (Pennington 1990, 1991). This woody family makes a large contribution to tropical plant biodiversity, being a major floristic component of tropical lowland wet forests in the Americas, Asia, Africa and the Pacific Islands (Gentry 1988). Sapotaceous plants are also economically important to humans. “Sapote” comes from the Nahuatl word meaning sweet fruit; most species bear such a fruit (e.g., the sapodilla and mamey sapote) (Smith et al. -

Following D U B a R D, the Considered Shape of the Embryo Is a Good Taxonomic Character
Notes on Guiana Sapotaceae by P. J. Eyma (Utrecht). the work Notwithstanding large amount of spent by several botanists this does not satis- on family, taxonomy appear very and has factory, a general agreement on generic limits not yet been reached. The result has been a perplexing number of generic and sectional The author for his names. present apologizes adding the number of to interpretations. This study of American Sapotaceae, primarily undertaken in connection with the Flora of Surinam, could not have been com- without the loan of the pleted generous specimens by herbaria at Brussels [B], Berlin —Dahlem [D], Kew [K], and Leyden [L]. the author short visit to the herbaria In 1934 paid a at Brussels [B] The collections and at Paris [P]. of this family at Paris are of special interest owing to the fact that they contain the material B i 11 Pierre and and studied by a on, Du'bard, bear numer- and ous notes analytical drawings, especially by Pierre, attached British to the sheets. A number of Guiana Sapotaceae from the Kew Herbarium was received for determination shortly afterwards. The author feels greatly indebted to the directors of the above their kind and mentioned Herbaria for hel{), particularly to Prof. Dr. P 11 Utrecht, under whose direction this A. u e, study was undertaken. Unless otherwise mentioned the specimens cited are in the Utrecht Herbarium [U]. The principial alterations in the classification of Sapotaceae in this due the for the paper are to rejection classifying purposes of certain number of flower-parts and, to a degree, of the staminodial development also. -

Pouteria Campechiana (Kunth) Baehni
Pouteria campechiana (Kunth) Baehni ANÍBAL NIEMBRO ROCAS Instituto de Ecología, A.C. Xalapa, Veracruz, México SAPOTACEAE (SAPODILLA FAMILY) No synonyms Canishte, k’anixté, mamey de Campeche, zapote amarillo, zapuyul Pouteria campechiana is native to America. It is distributed section, and 2 to 4 cm in diameter. The seedcoat is light brown naturally in Mexico and Central America, where it forms part in color, smooth, shiny, and osseous. It has a long and large lat- of the wet and subhumid tropical forests. eral hilum scar that is white or yellowish-cream in color and Pouteria campechiana is an evergreen tree that reaches occupies part of the body of the seed. 30 m in height and 30 cm at d.b.h. The trunk is straight with From August through October, the fruits are collected an irregular and dense crown, made up of thin and horizontal either directly from the ground or by climbing the trees and branches. The leaves are simple, alternate, gathered at the tips using poles with metal hooks. The pulp is removed from the of the branches, oblanceolate to oblanceolate-oblong, 6 to 25 pulpy fruits by hand inside a bucket of water. Resulting impu- cm long, and 2.5 to 8 cm wide. In the Yucatan Peninsula, the rities float and are gathered with a strainer. Good seeds sink. tree grows in calcareous soils with outcropping rocks, forming Subsequently, the seeds are dried in the sun in ventilated part of the tropical forest. The regions where the tree grows places for 1 or 2 hours, depending on the lighting conditions. -

Supplementary Material Saving Rainforests in the South Pacific
Australian Journal of Botany 65, 609–624 © CSIRO 2017 http://dx.doi.org/10.1071/BT17096_AC Supplementary material Saving rainforests in the South Pacific: challenges in ex situ conservation Karen D. SommervilleA,H, Bronwyn ClarkeB, Gunnar KeppelC,D, Craig McGillE, Zoe-Joy NewbyA, Sarah V. WyseF, Shelley A. JamesG and Catherine A. OffordA AThe Australian PlantBank, The Royal Botanic Gardens and Domain Trust, Mount Annan, NSW 2567, Australia. BThe Australian Tree Seed Centre, CSIRO, Canberra, ACT 2601, Australia. CSchool of Natural and Built Environments, University of South Australia, Adelaide, SA 5001, Australia DBiodiversity, Macroecology and Conservation Biogeography Group, Faculty of Forest Sciences, University of Göttingen, Büsgenweg 1, 37077 Göttingen, Germany. EInstitute of Agriculture and Environment, Massey University, Private Bag 11 222 Palmerston North 4474, New Zealand. FRoyal Botanic Gardens, Kew, Wakehurst Place, RH17 6TN, United Kingdom. GNational Herbarium of New South Wales, The Royal Botanic Gardens and Domain Trust, Sydney, NSW 2000, Australia. HCorresponding author. Email: [email protected] Table S1 (below) comprises a list of seed producing genera occurring in rainforest in Australia and various island groups in the South Pacific, along with any available information on the seed storage behaviour of species in those genera. Note that the list of genera is not exhaustive and the absence of a genus from a particular island group simply means that no reference was found to its occurrence in rainforest habitat in the references used (i.e. the genus may still be present in rainforest or may occur in that locality in other habitats). As the definition of rainforest can vary considerably among localities, for the purpose of this paper we considered rainforests to be terrestrial forest communities, composed largely of evergreen species, with a tree canopy that is closed for either the entire year or during the wet season. -

Gambeya Korupensis (Sapotaceae: Chrysophylloideae), a New Rain Forest Tree Species from the Southwest Region in Cameroon
KEW BULLETIN (2016) 71:28 ISSN: 0075-5974 (print) DOI 10.1007/S12225-016-9633-X ISSN: 1874-933X (electronic) Gambeya korupensis (Sapotaceae: Chrysophylloideae), a new rain forest tree species from the Southwest Region in Cameroon Corneille E. N. Ewango1,2, David Kenfack3, Moses Nsanyi Sainge4, Duncan W. Thomas5 & Xander M. van der Burgt6 Summary. Gambeya korupensis Ewango & Kenfack (Sapotaceae: Chrysophylloideae), a new rain forest tree species from the Southwest Region in Cameroon, is described and illustrated. A distribution map is provided. G. korupensis has the leaf blade below pubescent on the midribs and secondary nerves, flowers with a pedicel 0.5 – 1 mm long, and a fruit which is ovoid, attenuate at the apex, 5-ridged, verrucose between the ridges, and bright red at maturity. The conservation status of G. korupensis is assessed as Vulnerable according to IUCN criteria. Key Words. Chrysophyllum, conservation, IUCN Vulnerable, Korup National Park. Introduction 2006; Burgt 2009; Ewango & Breteler 2001; Kenfack Tropical forests inspire botanists and ecologists et al. 2004). The collections were also compared with because of their high diversity and the numerous authoritatively named material of all tropical African species still to be described. Great interest has been species of Gambeya in various herbaria (mostly still aroused by the likely impact of climate change and stored under Chrysophyllum L.; see below). The species fi development on their species diversity and more effort was identi ed as new and provisionally named as Tulestea is needed to document poorly known areas of sp. nov. based on fruit structure by D. W. Thomas biodiversity conservation priority, before their species (Thomas et al. -

Caractéristiques Floristiques De La Zone De Prony À Goro
RAPPORT DE CONSULTANCE CARACTÉRISTIQUES FLORISTIQUES DE LA ZONE DE PRONY À GORO Prilchardiopsis Jeanneneyi Instllut de reeherelTa pour le delleloppemem Laboratoire de Botanique et d'Écologie Appliquée Rapport établi par Tanguy JAFFRÉ (avec la collaboration de F. RIGAULT et G. DAGOSTINI) Juillet 2000 • • • • • • CARACTERISTIQUES FLORISTIQUES DE LA ZONE DE • PRONY A GORO • • • Tanguy JatTré, IRD, Centre de Nouméa. • (Avec la coilaboralion de F. Rigault el de G. Dagoslini) • • • • Méthode de travail Le temps très court imparti pour cette étude, à une période où peu de plantes sont en • fleurs, nous a permis de réaliser seulement un inventaire floristique partiel de la zone d'étude. Aussi avons-nous basé davantage notre analyse sur les récoltes anciennes que sur celles • etTectuées fin mai courant juin, au cours des sorties sur le terrain, réalisées par l'IRD et ia • STRAS. Nous avons également pris en compte les données floristiques laissées par S. McCoy, • lors de ses passages à l'herbier pour identification d'échantillons. Les prospections que nous avons réalisées ont consisté principalement à parcourir • quelques biotopes représentatifs, situés en bordure des pistes principales de la partie centrale de la zone d'étude. Nous nous sommes égaiement rendus dans les parties basses des réserves • botaniques du Pic du Grand Kaori, et du Mt Oungoné, ainsi que dans la zone sur gabbros de la • Baie Nord. Un survol de la zone d'étude en hélicoptère, avec quelques arrêts, nous a permis • d'avoir accès à des secteurs peu connus de Port Boisé et de la Kuébini. Nous avons recherché les informations, dans les 22 volumes de la Flore de la • Nouvelle-Calédonie (Aubréville & al. -

Floristic Relationships of New Caledonian Rainforest Phanerogams
Extract from Telopea 2(6): 631-679 (1986) 63 1 FLORISTIC RELATIONSHIPS OF NEW CALEDONIAN RAINFOREST PHANEROGAMS PH.MORAT!, J.-M. VELLONI& H. S. MACKEE~ (Accepted for publication 16.9.1983) ABSTRACT Morat, Ph.’, Veìllon, J.-M.’ & MacKee, H. S.2 (‘Centre ORSTOM, B.P. A5 Cedex, Nouméa, New Caledonia; 2L3.P. 3349, Nouméa, New Caledonia) 1984. Floristic relatiomhips of New Caledonian rainforest phanerogams. Telopea 2[4): 631-679 - A detailed analysis of the New Caledonian rainforest flora is given; 1499 species in 365 genera and 108 families are listed. Distribution of the species within New Caledonia is given in terms of specificity to rainforest (forestInon-forest and forest occurrence) and to substrate (only ultrabasiclabsent from ultrabasic/present on ultrabasic and other substr: :ss). Distribution of genera is presented according’to occurrences in 12 phyto- geographic units from endemic to pantropical. Sources of information are given. Comparisons with the whole New Caledonian phanerogamic flora are made; 46% of genera and species and 66% of families occur in the rainforest. For the flora the level of specific endemism is c. 75%. Floristic affinities are assessed by: comparison of numbers of genera shared with other regions (pantropical genera included/excluded); and numbers of genera shared exclusively by New Caledonia and 2, 3, 4, 5 or 6 other regions. In these comparisons Australia, New Guinea, Malesia, Fiji, the New Hebrides, the Solomon Islands and then New Zealand have the most genera in common with New Caledonia. A floristic affinity Co-efficient for each territory was calculated from the proportion of the number of common genera to the number of territories in which they occur, for groups of two to six territories.