Snake Harassment in the Cape Ground Squirrel (Xerus Inauris): Variation in Anti-Predator Behaviours, Predator Discrimination and Venom Resistance in A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Cultural Affairs and Sport Annual Report 2011/2012
Department of Cultural Affairs and Sport Annual Report 2011/2012 Department of Cultural Affairs and Sport – Annual Report 2011/2012 Dr IH Meyer Western Cape Minister of Cultural Affairs, Sport and Recreation I have the honour of submitting the Annual Report of the Department of Cultural Affairs and Sport for the period 1 April 2011 to 31 March 2012. _______________ BRENT WALTERS 31 August 2012 Department of Cultural Affairs and Sport – Annual Report 2011/2012 Contents PART 1: GENERAL INFORMATION .................................................................................................................................. 1 1.1 Vision, mission and values ............................................................................................................................. 1 1.2 Organisational structure ................................................................................................................................ 2 1.3 Legislative mandate ...................................................................................................................................... 3 1.4 Entities reporting to the Minister ................................................................................................................... 8 1.5 Minister’s statement ....................................................................................................................................... 9 1.6 Accounting Officer’s overview ................................................................................................................. -

Operational Environmental Management Programme (OEMP)
PO Box 12697 Die Boord, 7613 Consulting Environmentalists Phone/fax 021 8864056 Konsulterende Omgewingskundiges E-mail: [email protected] Operational Environmental Management Programme (OEMP) Revised Version 2012 16 NOVEMBER 2012 Prepared by: Ecosense cc Members: M. B. Sasman, M.I. Sasman, K.Myburgh, C. Rabie www.ecosense.co.za Atlantic Beach Estate Operational Phase Environmental Management Plan TABLE OF CONTENTS 1 CONTEXTUAL INFORMATION .................................................................................................................. 1 1.1 INTRODUCTION / BACKGROUND ........................................................................................................ 1 1.3 OBJECTIVES OF THE OEMP .................................................................................................................. 5 1.4 FORMAT OF THIS DOCUMENT ............................................................................................................. 5 2 DESCRIPTION OF THE ENVIRONMENT AND ANTICIPATED IMPACTS ........................................................ 6 2.1 CLIMATE ........................................................................................................................................... 6 2.2 GEOLOGY, SOILS AND WATER SOURCES ................................................................................. 6 2.3 TOPOGRAPHY AND DRAINAGE ................................................................................................... 6 2.4 FLORA ............................................................................................................................................... -

Fasanbi SHOWCASE
Threatened Species Monitoring PROGRAMME Threatened Species in South Africa: A review of the South African National Biodiversity Institutes’ Threatened Species Programme: 2004–2009 Acronyms ADU – Animal Demography Unit ARC – Agricultural Research Council BASH – Big Atlassing Summer Holiday BIRP – Birds in Reserves Project BMP – Biodiversity Management Plan BMP-S – Biodiversity Management Plans for Species CFR – Cape Floristic Region CITES – Convention on International Trade in Endangered Species CoCT – City of Cape Town CREW – Custodians of Rare and Endangered Wildflowers CWAC – Co-ordinated Waterbird Counts DEA – Department of Environmental Affairs DeJaVU – December January Atlassing Vacation Unlimited EIA – Environmental Impact Assessment EMI – Environmental Management Inspector GBIF – Global Biodiversity Information Facility GIS – Geographic Information Systems IAIA – International Association for Impact Assessment IAIAsa – International Association for Impact Assessment South Africa IUCN – International Union for Conservation of Nature LAMP – Long Autumn Migration Project LepSoc – Lepidopterists’ Society of Africa MCM – Marine and Coastal Management MOA – memorandum of agreement MOU – memorandum of understanding NBI – National Botanical Institute NEMA – National Environmental Management Act NEMBA – National Environmental Management Biodiversity Act NGO – non-governmental organization NORAD – Norwegian Agency for Development Co–operation QDGS – quarter-degree grid square SABAP – Southern African Bird Atlas Project SABCA – Southern African -

CSA Schools T20 Challenge 2 Pretoria | 6-8 March 2020 Messages
Messages Previous Winners Umpires Emergency Contacts Daily Programme Fixtures NATIONAL CRICKET WEEK POOL A | Team Lists POOL B | Team Lists Playing Conditions CSA SCHOOLS T20 Procedure for the Super Over T20 CHALLENGE Appendix 1 Pretoria | 6-8 March 2020 Appendix 2 Schools Code of Conduct Messages Chris Nenzani | President, Cricket South Africa Previous Winners Umpires The Schools’ T20 tournament CSA values our investment in youth extremely highly. It is is not just the biggest event an important contribution to nation building through cultural Emergency Contacts that Cricket South Africa (CSA) diversity which has become one of the pillars on which our has ever handled but it creates cricket is built. CSA has travelled a wonderful journey over the Daily Programme a pathway of opportunity for past 29 years of unity and everybody can be proud of his or her schools at all levels to live their contribution. dreams. Fixtures There are countless cricketers who have gone on from our It takes the game to every corner youth programs to engrave their names with distinction in South of the country and to established African cricket history and we congratulate them and thank them POOL A | Team Lists cricket schools as well as those that are just starting to make for their contributions. their way. As such it is a key component of our development POOL B | Team Lists program and of our vision and commitment to take the game to I must also put on record our thanks to all the people who have given up their time without reward to coach and mentor our all. -

Botswana Has Fifty Eight Different Types of Snakes
DANGEROUS SNAKES OF B OT SWA NA Botswana has fifty eight different types of snakes. Twenty two species are not venomous, while seven can inflict rather painful bites. Nine VERY DANGEROUS species are considered potentially deadly. DANGEROUS Has caused Painful bite, but does human fatalities not require antivenom VERY VERY VERY VERY DANGEROUS DANGEROUS DANGEROUS DANGEROUS Black Mamba Black Mamba Snouted Cobra Snouted Cobra - banded phase (Dendroaspis polylepis) (Dendroaspis polylepis) (Naja annulifera) (Naja annulifera) VERY VERY VERY VERY DANGEROUS DANGEROUS DANGEROUS DANGEROUS Anchieta’s Cobra Cape Cobra Cape Cobra Cape Cobra - juvenile (Naja anchietae) (Naja nivea) (Naja nivea) (Naja nivea) Photo Marius Burger VERY VERY VERY VERY DANGEROUS DANGEROUS DANGEROUS DANGEROUS Mozambique Spitting Cobra Common Boomslang - male Common Boomslang - female Common Boomslang - juvenile (Naja mossambica) (Dispholidus typus viridis) (Dispholidus typus viridis) Photo André Coetzer (Dispholidus typus viridis) VERY VERY DANGEROUS DANGEROUS DANGEROUS DANGEROUS Southern Twig Snake Puff Adder Horned Adder Bibron’s Stiletto Snake (Thelotornis capensis capensis) (Bitis arietans arietans) (Bitis caudalis) (Atractaspis bibronii) Photo Warren Dick © Johan Marais African Snakebite Institute Snakebite African © Johan Marais JOHAN MARAIS is the author of various books on reptiles including the best-seller A Complete Guide to Snakes of Southern Africa. He is a popular public speaker and offers a variety of courses including Snake Awareness, Scorpion Awareness EMERGENCY PROTOCOL and Venomous Snake Handling. Johan is accredited by the International Society of Zoological Sciences (ISZS) and is a IN THE EVENT OF A SNAKE BITE Field Guides Association of Southern Africa (FGASA) and DO NOT ww Travel Doctor-approved service provider. His courses are 1 Keep the victim calm, immobilized and .. -

Lenert Van Wyk
Lenert van Wyk Lenert van Wyk is a South African cricketer who currently plays for Boland. He is a right-handed upper-order batsman and part-time medium-fast bowler and has also played for the Cape Cobras. Jonah Lenert. Person, Person Or Being In Fiction. Jonah Lenert is the son of actress Rachel Chagall. Eve Lenert. Person, Person Or Being In Fiction. The latest Tweets from Lenert van Wyk (@LenertvanWyk). Married to the love of my life Risa van Wyk, Logistics Manager at Unichoice Produce Direct in Cape Town, ex Cricketer , Bcom Bachelors Degree @ Stellies!. Somerset West, South Africa. Lenert van Wyk was born in 1989. 1 person found this useful. Analysis of In detention by Christopher van Wyk? consider the philosophy of the bikers. Share to: When was Marguerite Lenert born? Marguerite Lenert was born on November 27, 1918, in United States. Share to: When was Coenie van Wyk born? Coenie van Wyk was born on 1988-01-08. Share to: When was Piet van Wyk de Vries born? Piet van Wyk de Vries was born on 1972-05-07. Share to: When was Abraham Erasmus van Wyk born? Lenert van Wyk (born 13 July 1989) is a South African cricketer who currently plays for Boland. He is a right-handed upper-order batsman and part-time medium-fast bowler and has also played for the Cape Cobras. The contents of this page are sourced from a Wikipedia article. The contents are available under the CC BY-SA 4.0 license. comment(s) so far. Leave a comment. -

Quantitative Characterization of the Hemorrhagic, Necrotic, Coagulation
Hindawi Journal of Toxicology Volume 2018, Article ID 6940798, 8 pages https://doi.org/10.1155/2018/6940798 Research Article Quantitative Characterization of the Hemorrhagic, Necrotic, Coagulation-Altering Properties and Edema-Forming Effects of Zebra Snake (Naja nigricincta nigricincta)Venom Erick Kandiwa,1 Borden Mushonga,1 Alaster Samkange ,1 and Ezequiel Fabiano2 1 School of Veterinary Medicine, Faculty of Agriculture and Natural Resources, Neudamm Campus, University of Namibia, P. Bag 13301, Pioneers Park, Windhoek, Namibia 2Department of Wildlife Management and Ecotourism, Katima Mulilo Campus, Faculty of Agriculture and Natural Resources, University of Namibia, P. Bag 1096, Ngweze, Katima Mulilo, Namibia Correspondence should be addressed to Alaster Samkange; [email protected] Received 30 May 2018; Revised 5 October 2018; Accepted 10 October 2018; Published 24 October 2018 Academic Editor: Anthony DeCaprio Copyright © 2018 Erick Kandiwa et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Tis study was designed to investigate the cytotoxicity and haemotoxicity of the Western barred (zebra) spitting cobra (Naja nigricincta nigricincta) venom to help explain atypical and inconsistent reports on syndromes by Namibian physicians treating victims of human ophidian accidents. Freeze-dried venom milked from adult zebra snakes was dissolved in phosphate bufered saline (PBS) for use in this study. Haemorrhagic and necrotic activity of venom were studied in New Zealand albino rabbits. Oedema-forming activity was investigated in 10-day-old Cobb500 broiler chicks. Procoagulant and thrombolytic activity was investigated in adult Kalahari red goat blood in vitro. -

A Preliminary Herpetological Survey of the Vilanculos Coastal Wildlife Sanctuary on the San Sebastian Peninsula, Vilankulo, Mozambique
Herpetology Notes, volume 3: 181-193 (2010) (published online on 31 May 2010) A preliminary herpetological survey of the Vilanculos Coastal Wildlife Sanctuary on the San Sebastian Peninsula, Vilankulo, Mozambique Niels H.G. Jacobsen1*, Errol W. Pietersen2 & Darren W. Pietersen3 Abstract. This paper reports on and discusses the findings of a herpetofaunal survey of the San Sebastian Peninsula, Vilankulo, Mozambique. A total of 39 reptile and 20 amphibian species were recorded including new records for Mozambique, range extensions and taxa previously considered endemic to the Bazaruto Archipelago. Keywords. Herpetofauna, San Sebastian Peninsula, Vilankulo, Mozambique. Introduction These islands form a northward extension of the San Sebastian Peninsula. The herpetofauna of Mozambique is still relatively A survey of the herpetofauna of the San Sebastian poorly known, especially when compared to the rest of Peninsula was undertaken as part of a larger study of southern Africa. The most recent accounts are those of the vegetation and fauna to assess the conservation Broadley (1966a, 1983), Poynton & Broadley (1985a, importance of the area. b, 1987, 1988) and Channing 2001. In addition, it appears that some early records have been overlooked The Study Site in museum collections. Apart from these, most The San Sebastian Peninsula lies south-east of the recent records often emanate from scant, sporadic town of Vilankulo, forming the mainland extension of or opportunistic collecting (Downs & Wirminghaus the Bazaruto Archipelago which includes Margaruque, 1990). As a result there is a void in our knowledge, Benguera, Bazaruto and Santa Carolina islands (Fig. 1). which also complicates the interpretation of species’ The Vilanculos Coastal Wildlife Sanctuary (VCWS) distributions and even the taxonomic status of some lies along the peninsula between 22.0833 and 22.3500° species. -
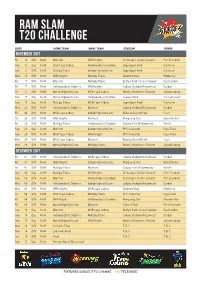
Ram Slam T20 CHALLENGE
RAm slam T20 CHALLENGE DATE HOME TEAM AWAY TEAM STADIUM VENUE November 2017 Fri 10 D/N 18:00 Warriors VKB Knights St George’s Cricket Ground Port Elizabeth Sun 12 Day 12:00 WSB Cape Cobras Hollywoodbets Dolphins SuperSport Park Centurion Sun 12 D/N 16:00 Multiply Titans bizhub Highveld Lions SuperSport Park Centurion Wed 15 D/N 18:00 VKB Knights Multiply Titans Diamond Oval Kimberley Fri 17 D/N 18:00 Warriors Multiply Titans Buffalo Park Cricket Stadium East London Fri 17 D/N 18:00 Hollywoodbets Dolphins VKB Knights Sahara Stadium Kingsmead Durban Fri 17 D/N 18:00 bizhub Highveld Lions WSB Cape Cobras Bidvest Wanderers Stadium Johannesburg Sun 19 Day 14:30 bizhub Highveld Lions Hollywoodbets Dolphins Senwes Park Potchefstroom Sun 19 Day 14:30 Multiply Titans WSB Cape Cobras SuperSport Park Centurion Wed 22 D/N 18:00 Hollywoodbets Dolphins Warriors Sahara Stadium Kingsmead Durban Fri 24 D/N 18:00 WSB Cape Cobras bizhub Highveld Lions Eurolux Boland Park Paarl Fri 24 D/N 18:00 VKB Knights Warriors Mangaung Oval Bloemfontein Fri 24 D/N 18:00 Multiply Titans Hollywoodbets Dolphins Sahara Park Willowmoore Benoni Sun 26 Day 12:00 Warriors bizhub Highveld Lions PPC Newlands Cape Town Sun 26 D/N 16:00 WSB Cape Cobras VKB Knights PPC Newlands Cape Town Wed 29 D/N 18:00 WSB Cape Cobras Warriors Eurolux Boland Park Paarl Wed 29 D/N 18:00 bizhub Highveld Lions Multiply Titans Bidvest Wanderers Stadium Johannesburg December 2017 Fri 01 D/N 18:00 Hollywoodbets Dolphins WSB Cape Cobras Sahara Stadium Kingsmead Durban Fri 01 D/N 18:00 VKB Knights bizhub -

Proposed Riviera Tungsten Project Magisterial District of Piketberg Western Cape Province
PROPOSED RIVIERA TUNGSTEN PROJECT MAGISTERIAL DISTRICT OF PIKETBERG WESTERN CAPE PROVINCE FINAL SCOPING REPORT REFERENCE NUMBER: WC 30/5/1/2/2/10110 MR FEBRUARY 2019 PREPARED FOR: PREPARED BY: Bongani Minerals (Pty) Ltd Greenmined Environmental Suite 2.1 On the Greens Unit MO1, No 36 AECI site Golf Village Baker Square, Paardevlei De Beers Avenue De Beers Avenue Somerset West Somerset West 7130 7130 Contact Person: Mr L Koster Contact Person: Ms C Fouche Tel: 060 785 2780 Tel: 021 851 2673 Cell: 083 265 7755 Cell: 082 811 8514 E-mail: [email protected] Fax: 086 546 0579 [email protected] RIVIERA TUNGSTEN FINAL SCOPING REPORT - FEBRUARY 2019 EXECUTIVE SUMMARY The Applicant, Bongani Minerals (Pty) Ltd, applied for environmental authorisation to mine tungsten and molybdenum from a 531.4405 ha area that extends over Portion 1 of Farm 297 RD, Portion 6 (Remaining Extent) of the farm Namaquasfontein 76 RD, and Portion 21 of the farm Namaquasfontein 76 RD. Greenmined will at all times remain independent and will perform its obligations in terms of all relevant Acts, Regulations and Guidelines, as expected from environmental practitioners. All documentation, to date, was based on preliminary data and desktop studies as access to the study area was denied by the landowners, resulting in limited information being provided to all commenting parties. Numerous attempts and letters requesting access to the properties by the applicant was all in vain. Greenmined is unable to provide the I&AP’s and stakeholders with material information with regards to this mining right application and it is therefore clear that the relevant authorities will not be able to make an informed decision, irrespective should it be positive or negative. -

Taxonomic Status of Cobras of the Genus Naja Laurenti (Serpentes: Elapidae)
Zootaxa 2236: 26–36 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) In praise of subgenera: taxonomic status of cobras of the genus Naja Laurenti (Serpentes: Elapidae) VAN WALLACH1, 4, WOLFGANG WÜSTER2 & DONALD G. BROADLEY3 1Museum of Comparative Zoology, Harvard University, Cambridge MA 02138, USA. E-mail: [email protected] 2School of Biological Sciences, Bangor University, Bangor LL57 2UW, UK. E-mail: [email protected] 3Biodiversity Foundation for Africa, P.O. Box FM 730, Famona, Bulawayo, Zimbabwe. E-mail: [email protected] 4corresponding author Abstract The genus Naja Laurenti, 1768, is partitioned into four subgenera. The typical form is restricted to 11 Asian species. The name Uraeus Wagler, 1830, is revived for a group of four non-spitting cobras inhabiting savannas and open formations of Africa and Arabia, while Boulengerina Dollo, 1886, is applied to four non-spitting African species of forest cobras, including terrestrial, aquatic and semi-fossorial forms. A new subgenus is erected for seven species of African spitting cobras. We recommend the subgenus rank as a way of maximising the phylogenetic information content of classifications while retaining nomenclatural stability. Key words: Naja, Uraeus, Boulengerina, Afronaja subgen. nov., taxonomy, Africa, Asia Introduction The scientific nomenclature of life serves the key function of providing labels for the cataloguing of the Earth’s biodiversity and thus for information retrieval. In order to make a system of classification predictive, it is generally agreed that a classification should reflect the current state of knowledge about the evolutionary relationships within a group, which, in the case of a nested, hierarchical system of nomenclature, means recognizing only monophyletic groups as named taxa. -

LABOUR REPORT Pride • Unity • Trust QUARTER 3 of 2018
Affiliated to Fedusa LABOUR REPORT www.untu.co.za pride • unity • trust QUARTER 3 OF 2018 PAGE 4 PAGE 11 PAGE 12 PAGE 16 RAILWAY SAFETY REGULATOR LABOUR ORGANISASION ASKED “VOETPLAATPARK IN KZN ENSURING COMPLIANCE IN SA TO GUIDE UNION ON DAGGA PRASA’S MODERNISATION FLOP IS UNTU’S BEST BENEFIT” Cyril Ramaphosa yet to deliver on promise to turn struggling SOE ship around State failing members, taxpayers hree quarters after the ap- tect the assets of Prasa bers at the RSR and is on the pended, the fate of Gama remains uncertain. pointment of President Cyril which belongs to millions verge of obtaining recognition. The allegations against them are related Ramaphosa, South Africans of taxpayers. The Union has been locking to a multibillion-rand procurement deal for have yet to see his promised “The Auditor- horns with the RSR after it over a thousand locomotives emanating improvements to the poor- General (AG) added granted Prasa a safety operating from a transaction in 2014. Tly governed State-Owned Enterprises to Nzimande’s plate permit for another year till Evans says Molefe was at the steer of (SOE’s) in our country. Although UNTU when he qualified Prasa’s August 2019. the Board of Prasa during the period of realises Rome was not built in a day, too late financial statements Harris says the Union the AG’s shocking financial report. little is being done too late putting the jobs for R20,3 billion in irregular was disturbed to learn “To date, nothing has come of the cor- of our 37 000 members at risk.