Elasmobranch Fisheries Management Techniques 2004
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AC26 Inf. 1 (English Only / Únicamente En Inglés / Seulement En Anglais)
AC26 Inf. 1 (English only / únicamente en inglés / seulement en anglais) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-sixth meeting of the Animals Committee Geneva (Switzerland), 15-20 March 2012 and Dublin (Ireland), 22-24 March 2012 RESPONSE TO NOTIFICATION TO THE PARTIES NO. 2011/049, CONCERNING SHARKS The attached information document has been submitted by the Secretariat at the request of PEW, in relation to agenda item 16*. * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat or the United Nations Environment Programme concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. AC26 Inf. 1 – p. 1 January 5, 2012 Pew Environment Group Response to CITES Notification 2011/049 To Whom it May Concern, As an active international observer to CITES, a member of the Animals Committee Shark Working Group, as well as other working groups of the Animals and Standing Committees, and an organization that is very active in global shark conservation, the Pew Environment Group submits the following information in response to CITES Notification 2011/049. We submit this information in an effort to ensure a more complete response to the request for information, especially considering that some countries that have adopted proactive new shark conservation policies are not Parties to CITES. 1. Shark species which require additional action In response to Section a) ii) of the Notification, the Pew Environment Group submits the following list of shark species requiring additional action to enhance their conservation and management. -

Redalyc.Population Structure of the Pacific Angel Shark (Squatina Californica) Along the Northwestern Coast of Mexico Based on T
Ciencias Marinas ISSN: 0185-3880 [email protected] Universidad Autónoma de Baja California México Ramírez-Amaro, Sergio; Ramírez-Macías, Dení; Vázquez-Juárez, Ricardo; Flores- Ramírez, Sergio; Galván-Magaña, Felipe; Gutiérrez-Rivera, Jesús N. Population structure of the Pacific angel shark (Squatina californica) along the northwestern coast of Mexico based on the mitochondrial DNA control region Ciencias Marinas, vol. 43, núm. 1, 2017, pp. 69-80 Universidad Autónoma de Baja California Ensenada, México Available in: http://www.redalyc.org/articulo.oa?id=48050568004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Ciencias Marinas (2017), 43(1): 69–80 http://dx.doi.org/10.7773/cm.v43i1.2692 Population structure of the Pacific angel shark (Squatina californica) along the northwestern coast of Mexico based on the mitochondrial DNA control region Estructura poblacional del tiburón ángel del Pacífico (Squatina californica) a lo largo de la costa noroccidental de México con base en la regíon control del ADN mitocondrial Sergio Ramírez-Amaro1, Dení Ramírez-Macías2, Ricardo Vázquez-Juárez3*, Sergio Flores-Ramírez4, Felipe Galván-Magaña5, Jesús N Gutiérrez-Rivera3 1 Universitat de les Illes Balears, Laboratori de Genètica, Carretera de Valldemossa km 7.5, 07122, Palma de Mallorca, Spain. 2 Tiburón Ballena México de ConCIENCIA México, Manatí 4, no. 802, colonia Esperanza III, CP 23090, La Paz, Baja California Sur, México. 3 Centro de Investigaciones Biológicas del Noroeste, Mar Bermejo 195, colonia Playa Palo de Santa Rita Sur, CP 23096, La Paz, Baja California Sur, México. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Proposal for Inclusion of the Angelshark
CMS Distribution: General CONVENTION ON UNEP/CMS/COP12/Doc.25.1.23 MIGRATORY 6 June 2017 SPECIES Original: English 12th MEETING OF THE CONFERENCE OF THE PARTIES Manila, Philippines, 23 - 28 October 2017 Agenda Item 25.1. PROPOSAL FOR THE INCLUSION OF THE ANGELSHARK (Squatina squatina) ON APPENDIX I AND II OF THE CONVENTION Summary: The Government of Monaco has submitted the attached proposal* for the inclusion of the Angelshark (Squatina squatina) on Appendix I and II of CMS. *The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CMS Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. UNEP/CMS/COP12/Doc.25.1.23 PROPOSAL FOR THE INCLUSION OF THE ANGELSHARK (Squatina squatina) ON THE APPENDICES OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS A. PROPOSAL: Inclusion of the species Squatina squatina, Angelshark, in Appendix I and II. B. PROPONENT: Government of the Principality of Monaco C. SUPPORTING STATEMENT 1. Taxonomy 1.1 Class: Chondrichthyes, subclass Elasmobranchii 1.2 Order: Squatiniformes 1.3 Family: S quatinidae 1.4 Genus, species: Squatina squatina Linnaeus, 1758 1.5 Scientific synonyms 1.6 Common Name: English: Angelshark, common or European Angelshark, angel ray, shark ray, monkfish French: Ange de mer commun, L'angelot, Spanish: Angelote, Peje angel, tiburón angel German: Meerengel, Engelhai, Gemeiner Meerengel Italian: Angelu, Pesce angelo, Squatru cefalu, Terrezzino Portuguese: Anjo, Peixe anjo, Viola 2. -

Length at Maturity of the Pacific Angel Shark (Squatina Californica) in the Artisanal Elasmobranch Fishery in the Gulf Of
359 National Marine Fisheries Service Fishery Bulletin First U.S. Commissioner established in 1881 of Fisheries and founder NOAA of Fishery Bulletin Abstract—Median length at maturity Length at maturity of the Pacific angel was determined for Pacific angel sharks (Squatina californica) captured inciden- shark (Squatina californica) in the artisanal tally in the artisanal elasmobranch fish- ery in the Gulf of California in Mexico. elasmobranch fishery in the Gulf A total of 306 Pacific angel sharks (192 of California in Mexico females and 114 males) were analyzed. The size of the females ranged between 23 and 100 cm total length (TL), J. Fernando Márquez-Farías whereas males ranged between 25 and 99 cm TL. The maturity stages of both Email address for contact author: [email protected] females and males were determined by using the development of internal and Facultad de Ciencias del Mar external organs. The results of analysis Universidad Autónoma de Sinaloa of covariance reveal a significant effect Paseo Claussen s/n of sex (P<0.001) on length at maturity. A Col. Los Pinos binary logistic regression was applied for 82000 Mazatlán, Sinaloa, Mexico each sex to estimate the length at which half of the individuals were consid- ered mature (L50). For females, L50 was 74.41 cm TL (95% confidence interval [CI]: 72.81–76.00 cm TL), and the steep- ness coefficient (Φ) was 1.43 (95% CI: 0.84–2.42). For males, L50 was 77.82 cm The Pacific angel shark (Squatina Peninsula (BCP) in Mexico . Biological TL (95% CI: 75.66–79.97 cm TL), and californica) belongs to the family Squa- information about the Pacific angel the Φ was 3.08 (95% CI: 1.98–4.77). -

Wk Shark Advice Adhshark
ICES Special Request Advice Ecoregions in the Northeast Atlantic and adjacent seas Published 25 September 2020 NEAFC and OSPAR joint request on the status and distribution of deep-water elasmobranchs Advice summary In response to a joint request from NEAFC and OSPAR, ICES reviewed existing information on deep-water sharks, skates and rays from surveys and the literature. Distribution maps were generated for 21 species, showing the location of catches from available survey data on deep-water sharks and elasmobranchs in the NEAFC and OSPAR areas of the Northeast Atlantic. Shapefiles of the species distribution areas are available as supporting documentation to this work. This advice sheet presents a summary of ICES advice on the stock status of species for which an assessment is available, as well as current knowledge on the stock status of species for which ICES does not provide advice. An overview of approaches which may be applied to mitigate bycatch and to improve stock status is also presented. ICES recognizes that, despite their limitations, prohibition, gear and depth limitations, and TAC are mechanisms currently available to managers to regulate outtake; therefore, ICES advises that these mechanisms should be maintained. Furthermore, ICES advises that additional measures, such as electromagnetic exclusion devices, acoustic or light-based deterrents, and spatio-temporal management could be explored. Request NEAFC and OSPAR requested ICES to produce: a. Maps and shapefiles of the distribution of the species, identifying, if possible, key areas used during particular periods/stages of the species’ lifecycle in terms of distribution and relative abundance of the species, and expert interpretation of the data products; b. -
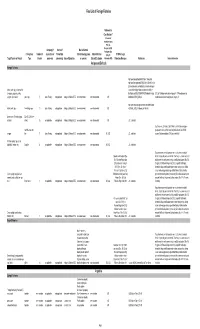
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Marine Protected Species Identification Guide
Department of Primary Industries and Regional Development Marine protected species identification guide June 2021 Fisheries Occasional Publication No. 129, June 2021. Prepared by K. Travaille and M. Hourston Cover: Hawksbill turtle (Eretmochelys imbricata). Photo: Matthew Pember. Illustrations © R.Swainston/www.anima.net.au Bird images donated by Important disclaimer The Chief Executive Officer of the Department of Primary Industries and Regional Development and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from the use or release of this information or any part of it. Department of Primary Industries and Regional Development Gordon Stephenson House 140 William Street PERTH WA 6000 Telephone: (08) 6551 4444 Website: dpird.wa.gov.au ABN: 18 951 343 745 ISSN: 1447 - 2058 (Print) ISBN: 978-1-877098-22-2 (Print) ISSN: 2206 - 0928 (Online) ISBN: 978-1-877098-23-9 (Online) Copyright © State of Western Australia (Department of Primary Industries and Regional Development), 2021. ii Marine protected species ID guide Contents About this guide �������������������������������������������������������������������������������������������1 Protected species legislation and international agreements 3 Reporting interactions ���������������������������������������������������������������������������������4 Marine mammals �����������������������������������������������������������������������������������������5 Relative size of cetaceans �������������������������������������������������������������������������5 -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -

Interstate Fisheries Management Plan for Atlantic Coastal Sharks
Fishery Management Report No. 46 of the Atlantic States Marine Fisheries Commission Working towards healthy, self-sustaining populations for all Atlantic coast fish species or successful restoration well in progress by the year 2015. Interstate Fishery Management Plan for Atlantic Coastal Sharks August 2008 Fishery Management Report No. 46 of the ATLANTIC STATES MARINE FISHERIES COMMISSION Interstate Fishery Management Plan for Atlantic Coastal Sharks August 2008 i Interstate Fishery Management Plan for Atlantic Coastal Sharks Prepared by Atlantic States Marine Fisheries Commission Coastal Sharks Plan Development Team Plan Development Team Members: Christopher M. Vonderweidt (Atlantic States Marine Fisheries Commission, PDT Chair), Karyl Brewster-Geisz (NOAA Fisheries Office of Sustainable Fisheries), Greg Skomal (Massachusetts Division of Marine Fisheries), Dr. Donna Fisher (Georgia Southern University), and Fritz Rohde (North Carolina Division of Marine Fisheries) Also Prepared by: Melissa Paine (ASMFC), Jessie Thomas (ASMFC) The Plan Development Team would like to thank the following people for assisting in the development this document: Robert Beal (ASMFC), LeAnn Southward Hogan (NOAA Fisheries Office of Sustainable Fisheries), Jack Musick (VIMS, TC Chair), Michael Howard (ASMFC), John Tulik (MA DLE) Toni Kerns (ASMFC), Nichola Meserve (ASMFC), Braddock Spear (ASMFC), Steve Meyers (NOAA Fisheries Office of Sustainable Fisheries), Russell Hudson (AP Chair), Claire McBane (NH DMF) This Management Plan was prepared under the guidance of the Atlantic States Marine Fisheries Commission’s Spiny Dogfish & Coastal Sharks Management Board, Chaired by Eric Smith of Connecticut. The Coastal Sharks Technical Committee, Advisory Panel, and Law Enforcement Committee provided technical and advisory assistance. This is a report of the Atlantic States Marine Fisheries Commission pursuant to U.S. -

A New Squalid Species of the Genus Centroscyllium from the Emperor Seamount Chain
魚 類 学 雑 誌 Japanese Journal of Ichthyology 36巻4号1990年 Vol.36, No.4 1990 A New Squalid Species of the Genus Centroscyllium from the Emperor Seamount Chain Shigeru Shirai and Kazuhiro Nakaya (Received January 26, 1989) Abstract A new etmopterine species, Centroscylliumexcelsum, is described from 21 adult speci- mens captured in the Emperor Seamount Chain, central North Pacific. The present species is distinguished from its congeners in having a very high and semicircular-shaped 1st dorsal fin with a developed spine, dermal denticles only on dorsal side of head and trunk, the 2nd dorsal spine arising behind pelvic fin base, a short caudal peduncle, and prefrontal wall and chondrified eye stalk on neurocranium. Ten embryos were collected from one of the female specimens, and some embryonic features are also noted. The genus Centroscyllium Muller et Henle is a is described below. small but well-defined assemblage. Among etmopterines, these Centroscyllium species are Methods and materials strikingly characterized by upper and lower jaw teeth similar in form, with 2 to 4 distinct lateral Methods for making external measurements cusps, arranged quincuncially; keel-process of follow Springer (1964) with several exceptions basal cranii extending anteroventrally from and additions as follows: origin of each dorsal fin suborbital region of neurocranium; subnasal is substituted by anterior point of emergence of stay at posterolateral edge of subnasal fenestra; each dorsal spine (see Krefft, 1968); distances from and unpaired genio-coracoideus directly origi- snout tip to nostrils and to mouth (preoral snout) nating from the frontal surface of coracoid sym- are measured, parallel to body axis, to posterior physis (Shirai and Nakaya, in press).