Century Paradigm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Long-Term Adult Human Brain Slice Cultures As a Model System to Study
TOOLS AND RESOURCES Long-term adult human brain slice cultures as a model system to study human CNS circuitry and disease Niklas Schwarz1, Betu¨ l Uysal1, Marc Welzer2,3, Jacqueline C Bahr1, Nikolas Layer1, Heidi Lo¨ ffler1, Kornelijus Stanaitis1, Harshad PA1, Yvonne G Weber1,4, Ulrike BS Hedrich1, Ju¨ rgen B Honegger4, Angelos Skodras2,3, Albert J Becker5, Thomas V Wuttke1,4†*, Henner Koch1†* 1Department of Neurology and Epileptology, Hertie-Institute for Clinical Brain Research, University of Tu¨ bingen, Tu¨ bingen, Germany; 2Department of Cellular Neurology, Hertie-Institute for Clinical Brain Research, University of Tu¨ bingen, Tu¨ bingen, Germany; 3German Center for Neurodegenerative Diseases (DZNE), Tu¨ bingen, Germany; 4Department of Neurosurgery, University of Tu¨ bingen, Tu¨ bingen, Germany; 5Department of Neuropathology, Section for Translational Epilepsy Research, University Bonn Medical Center, Bonn, Germany Abstract Most of our knowledge on human CNS circuitry and related disorders originates from model organisms. How well such data translate to the human CNS remains largely to be determined. Human brain slice cultures derived from neurosurgical resections may offer novel avenues to approach this translational gap. We now demonstrate robust preservation of the complex neuronal cytoarchitecture and electrophysiological properties of human pyramidal neurons *For correspondence: in long-term brain slice cultures. Further experiments delineate the optimal conditions for efficient [email protected] viral transduction of cultures, enabling ‘high throughput’ fluorescence-mediated 3D reconstruction tuebingen.de (TVW); of genetically targeted neurons at comparable quality to state-of-the-art biocytin fillings, and [email protected] demonstrate feasibility of long term live cell imaging of human cells in vitro. -
Thenerveimpulse05.Pdf
The nerve impulse. INTRODUCTION Axons are responsible for the transmission of information between different points of the nervous system and their function is analogous to the wires that connect different points in an electric circuit. However, this analogy cannot be pushed very far. In an electrical circuit the wire maintains both ends at the same electrical potential when it is a perfect conductor or it allows the passage of an electron current when it has electrical resistance. As we will see in these lectures, the axon, as it is part of a cell, separates its internal medium from the external medium with the plasma membrane and the signal conducted along the axon is a transient potential difference1 that appears across this membrane. This potential difference, or membrane potential, is the result of ionic gradients due to ionic concentration differences across the membrane and it is modified by ionic flow that produces ionic currents perpendicular to the membrane. These ionic currents give rise in turn to longitudinal currents closing local ionic current circuits that allow the regeneration of the membrane potential changes in a different region of the axon. This process is a true propagation instead of the conduction phenomenon occurring in wires. To understand this propagation we will study the electrical properties of axons, which include a description of the electrical properties of the membrane and how this membrane works in the cylindrical geometry of the axon. Much of our understanding of the ionic mechanisms responsible for the initiation and propagation of the action potential (AP) comes from studies on the squid giant axon by A. -

Spatial Extent of Neurons: Dendrites and Axons
Spatial extent of neurons: dendrites and axons Morphological diversity of neurons Cortical pyramidal cell Purkinje cell Retinal ganglion cells Motoneurons Dendrites: spiny vs non-spiny Recording and simulating dendrites Axons - myelinated vs unmyelinated Axons - myelinated vs unmyelinated • Myelinated axons: – Long-range axonal projections (motoneurons, long-range cortico-cortical connections in white matter, etc) – Saltatory conduction; – Fast propagation (10s of m/s) • Unmyelinated axons: – Most local axonal projections – Continuous conduction – Slower propagation (a few m/s) Recording from axons Recording from axons Recording from axons - where is the spike generated? Modeling neuronal processes as electrical cables • Axial current flowing along a neuronal cable due to voltage gradient: V (x + ∆x; t) − V (x; t) = −Ilong(x; t)RL ∆x = −I (x; t) r long πa2 L where – RL: total resistance of a cable of length ∆x and radius a; – rL: specific intracellular resistivity • ∆x ! 0: πa2 @V Ilong(x; t) = − (x; t) rL @x The cable equation • Current balance in a cylinder of width ∆x and radius a • Axial currents leaving/flowing into the cylinder πa2 @V @V Ilong(x + ∆x; t) − Ilong(x; t) = − (x + ∆x; t) − (x; t) rL @x @x • Ionic current(s) flowing into/out of the cell 2πa∆xIion(x; t) • Capacitive current @V I (x; t) = 2πa∆xc cap M @t • Kirschoff law Ilong(x + ∆x; t) − Ilong(x; t) + 2πa∆xIion(x; t) + Icap(x; t) = 0 • In the ∆x ! 0 limit: @V a @2V cM = 2 − Iion @t 2rL @x Compartmental appoach Compartmental approach Modeling passive dendrites: Cable equation -

The Narrowing of Dendrite Branches Across Nodes Follows a Well-Defined Scaling Law
The narrowing of dendrite branches across nodes follows a well-defined scaling law Maijia Liaoa, Xin Liangb, and Jonathon Howarda,1 aDepartment of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520; and bTsinghua-Peking Joint Center for Life Sciences, School of Life Sciences, Tsinghua University, 100084 Beijing, China Edited by Yuh Nung Jan, Howard Hughes Medical Institute, University of California San Francisco, San Francisco, CA, and approved May 17, 2021 (received for review October 28, 2020) The systematic variation of diameters in branched networks has minimized. Da Vinci’s law has been reformulated as the “pipe tantalized biologists since the discovery of da Vinci’s rule for trees. model” for plants, in which a fixed cross-section of stems and Da Vinci’s rule can be formulated as a power law with exponent branches is required to support each unit amount of leaves (13). two: The square of the mother branch’s diameter is equal to the Experimental support for scaling laws, however, is scarce because sum of the squares of those of the daughters. Power laws, with of the difficulties of imaging entire branched networks and because different exponents, have been proposed for branching in circula- intrinsic anatomical variability may obscure precise laws (14). Thus, tory systems (Murray’s law with exponent 3) and in neurons (Rall’s it is an open question whether scaling laws such as Eq. 1 apply in law with exponent 3/2). The laws have been derived theoretically, biological systems. based on optimality arguments, but, for the most part, have not To quantitatively test diameter-scaling laws in neurons, it is been tested rigorously. -

Structure and Function Relationship in Nerve Cells & Membrane Potential
Structure and Function Relationship in Nerve Cells & Membrane Potential Asist. Prof. Aslı AYKAÇ NEU Faculty of Medicine Biophysics Nervous System Cells • Glia – Not specialized for information transfer – Support neurons • Neurons (Nerve Cells) – Receive, process, and transmit information Neurons • Neuron Doctrine – The neuron is the functional unit of the nervous system • Specialized cell type – have very diverse in structure and function Neuron: Structure/Function • designed to receive, process, and transmit information – Dendrites: receive information from other neurons – Soma: “cell body,” contains necessary cellular machinery, signals integrated prior to axon hillock – Axon: transmits information to other cells (neurons, muscles, glands) • Information travels in one direction – Dendrite → soma → axon How do neurons work? • Function – Receive, process, and transmit information – Conduct unidirectional information transfer • Signals – Chemical – Electrical Membrane Potential Because of motion of positive and negative ions in the body, electric current generated by living tissues. What are these electrical signals? • receptor potentials • synaptic potentials • action potentials Why are these electrical signals important? These signals are all produced by temporary changes in the current flow into and out of cell that drives the electrical potential across the cell membrane away from its resting value. The resting membrane potential The electrical membrane potential across the membrane in the absence of signaling activity. Two type of ions channels in the membrane – Non gated channels: always open, important in maintaining the resting membrane potential – Gated channels: open/close (when the membrane is at rest, most gated channels are closed) Learning Objectives • How transient electrical signals are generated • Discuss how the nongated ion channels establish the resting potential. -
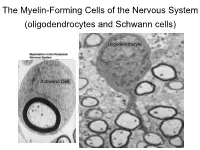
The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

NEURAL CONNECTIONS: Some You Use, Some You Lose
NEURAL CONNECTIONS: Some You Use, Some You Lose by JOHN T. BRUER SOURCE: Phi Delta Kappan 81 no4 264-77 D 1999 . The magazine publisher is the copyright holder of this article and it is reproduced with permission. Further reproduction of this article in violation of the copyright is prohibited JOHN T. BRUER is president of the James S. McDonnell Foundation, St. Louis. This article is adapted from his new book, The Myth of the First Three Years (Free Press, 1999), and is reprinted by arrangement with The Free Press, a division of Simon Schuster Inc. ©1999, John T. Bruer . OVER 20 YEARS AGO, neuroscientists discovered that humans and other animals experience a rapid increase in brain connectivity -- an exuberant burst of synapse formation -- early in development. They have studied this process most carefully in the brain's outer layer, or cortex, which is essentially our gray matter. In these studies, neuroscientists have documented that over our life spans the number of synapses per unit area or unit volume of cortical tissue changes, as does the number of synapses per neuron. Neuroscientists refer to the number of synapses per unit of cortical tissue as the brain's synaptic density. Over our lifetimes, our brain's synaptic density changes in an interesting, patterned way. This pattern of synaptic change and what it might mean is the first neurobiological strand of the Myth of the First Three Years. (The second strand of the Myth deals with the notion of critical periods, and the third takes up the matter of enriched, or complex, environments.) Popular discussions of the new brain science trade heavily on what happens to synapses during infancy and childhood. -

Gathering at the Nodes
RESEARCH HIGHLIGHTS GLIA Gathering at the nodes Saltatory conduction — the process by which that are found at the nodes of Ranvier, where action potentials propagate along myelinated they interact with Na+ channels. When the nerves — depends on the fact that voltage- authors either disrupted the localization of gated Na+ channels form clusters at the nodes gliomedin by using a soluble fusion protein of Ranvier, between sections of the myelin that contained the extracellular domain of sheath. New findings from Eshed et al. show neurofascin, or used RNA interference to that Schwann cells produce a protein called suppress the expression of gliomedin, the gliomedin, and that this is responsible for characteristic clustering of Na+ channels at the clustering of these channels. the nodes of Ranvier did not occur. The formation of the nodes of Ranvier is Aggregation of the domain of gliomedin specified by the myelinating cells, not the that binds neurofascin and NrCAM on the axons, and an important component of this surface of purified neurons also caused process in the peripheral nervous system the clustering of neurofascin, Na+ channels is the extension of microvilli by Schwann and other nodal proteins. These findings cells. These microvilli contact the axons at support a model in which gliomedin on the nodes, and it is here that the Schwann References and links Schwann cell microvilli binds to neurofascin ORIGINAL RESEARCH PAPER Eshed, Y. et al. Gliomedin cells express the newly discovered protein and NrCAM on axons, causing them to mediates Schwann cell–axon interaction and the molecular gliomedin. cluster at the nodes of Ranvier, and leading assembly of the nodes of Ranvier. -

How Do Dendrites Take Their Shape?
© 2001 Nature Publishing Group http://neurosci.nature.com review How do dendrites take their shape? Ethan K. Scott1 and Liqun Luo1,2 1 Department of Biological Sciences, Stanford University, Stanford, California 94305, USA 2 Neurosciences Program, Stanford University, Stanford, California 94305, USA Correspondence should be addressed to L.L. ([email protected]) Recent technical advances have made possible the visualization and genetic manipulation of individual dendritic trees. These studies have led to the identification and characterization of molecules that are important for different aspects of dendritic development. Although much remains to be learned, the existing knowledge has allowed us to take initial steps toward a comprehensive understanding of how complex dendritic trees are built. In this review, we describe recent advances in our understanding of the molecular mechanisms underlying dendritic morphogenesis, and discuss their cell-biological implications. With their great complexity and variety, dendrites (Fig. 1) are won- added advantage, can be used to study loss-of-function muta- ders of nature’s design. Built to receive and integrate inputs to neu- tions. Much of the progress reviewed in this article would not rons, dendrites occupy much of the brain’s volume and have been have been possible without these technological advances. the subject of studies since the days of Golgi and Cajal1. Over the course of much of the twentieth century, the prevailing belief that Breaking down complex dendritic trees axons take the more active role in wiring the brain and in estab- Although the processes used to build dendritic trees are complex lishing synaptic specificity led researchers to focus on the develop- and diverse (Fig. -

Dendrites and Disease Daniel Johnston, Andreas Frick, and Nicholas Poolos
OUP-FIRST UNCORRECTED PROOF, October 5, 2015 Chapter 24 Dendrites and disease Daniel Johnston, Andreas Frick, and Nicholas Poolos Summary While the functional properties of dendrites in the normal brain are the main focus of this book, there is a long history of research related to changes in dendrites that are associated with certain neurological, psychiatric, and developmental disorders. Much of this research has documented morphological changes in dendritic structure, but a significant increase in work has appeared since the second edition of this book in which changes in dendritic function have been described. In this chapter we briefly review some of the research relating structural changes in dendrites to disease, sufficient to provide a beginning reference source for readers interested in pursuing the subject further. The bulk of this chapter, however, will focus on the current state of knowledge related to dendritic “channelopathies.” These are defined as genetic or acquired defects in the normal func- tion or expression of ion channels (with a focus on voltage-gated ion channels) that are known to regulate how neuronal dendrites process and store information. The most specific and detailed information is available for epilepsy, which will be discussed at some length, but other data will be presented for certain neurodevelopmental disorders (e.g., autism, fragile X syndrome) and dis- eases of the adult and aging brain. Based on our knowledge of the normal properties of dendrites, we provide a framework for understanding how dendritic channelopathies can influence synaptic integration and plasticity and thus form the basis of abnormal brain function. Introduction Abnormalities in dendritic structure are a characteristic feature of many brain disorders. -

New Plasmon-Polariton Model of the Saltatory Conduction
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.17.910596; this version posted January 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Manuscript submitted to BiophysicalJournal Article New plasmon-polariton model of the saltatory conduction W. A. Jacak1,* ABSTRACT We propose a new model of the saltatory conduction in myelinated axons. This conduction of the action potential in myelinated axons does not agree with the conventional cable theory, though the latter has satisfactorily explained the electro- signaling in dendrites and in unmyelinated axons. By the development of the wave-type concept of ionic plasmon-polariton kinetics in axon cytosol we have achieved an agreement of the model with observed properties of the saltatory conduction. Some resulting consequences of the different electricity model in the white and the gray matter for nervous system organization have been also outlined. SIGNIFICANCE Most of axons in peripheral nervous system and in white matter of brain and spinal cord are myelinated with the action potential kinetics speed two orders greater than in dendrites and in unmyelinated axons. A decrease of the saltatory conduction velocity by only 10% ceases body functioning. Conventional cable theory, useful for dendrites and unmyelinated axon, does not explain the saltatory conduction (discrepancy between the speed assessed and the observed one is of one order of the magnitude). We propose a new nonlocal collective mechanism of ion density oscillations synchronized in the chain of myelinated segments of plasmon-polariton type, which is consistent with observations.