Changes in the Distribution of Tenascin During Tooth Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preparation of Absorption-Resistant Hard Tissue Using Dental Pulp-Derived Cells and Honeycomb Tricalcium Phosphate
materials Article Preparation of Absorption-Resistant Hard Tissue Using Dental Pulp-Derived Cells and Honeycomb Tricalcium Phosphate Kiyofumi Takabatake 1, Keisuke Nakano 1,* , Hotaka Kawai 1, Yasunori Inada 1, Shintaro Sukegawa 1,2 , Shan Qiusheng 1, Shigeko Fushimi 1, Hidetsugu Tsujigiwa 1,3 and Hitoshi Nagatsuka 1 1 Department of Oral Pathology and Medicine, Graduate School of Medicine, Dentistry and Pharmaceutical Science, Okayama University, Okayama 700-8525, Japan; [email protected] (K.T.); [email protected] (H.K.); [email protected] (Y.I.); [email protected] (S.S.); [email protected] (S.Q.); [email protected] (S.F.); [email protected] (H.T.); [email protected] (H.N.) 2 Department of Oral and Maxillofacial Surgery, Kagawa Prefectural Central Hospital, Kagawa 760-8557, Japan 3 Department of Life Science, Faculty of Science, Okayama University of Science, Okayama 700-0005, Japan * Correspondence: [email protected] Abstract: In recent years, there has been increasing interest in the treatment of bone defects using undifferentiated mesenchymal stem cells (MSCs) in vivo. Recently, dental pulp has been proposed as a promising source of pluripotent mesenchymal stem cells (MSCs), which can be used in various clinical applications. Dentin is the hard tissue that makes up teeth, and has the same composition and strength as bone. However, unlike bone, dentin is usually not remodeled under physiological conditions. Here, we generated odontoblast-like cells from mouse dental pulp stem cells and combined them with honeycomb tricalcium phosphate (TCP) with a 300 µm hole to create bone-like Citation: Takabatake, K.; Nakano, K.; tissue under the skin of mice. -

Collagen Fibres Are Not Required for Initial Matrix Mineralization by Bone Cells
Cells and Materials Volume 6 Number 1 Numbers 1-3 Article 23 1996 Collagen Fibres are Not Required for Initial Matrix Mineralization by Bone Cells M. M. Hosseini University of Toronto S. A. F. Peel University of Toronto J. E. Davies University of Toronto Follow this and additional works at: https://digitalcommons.usu.edu/cellsandmaterials Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Hosseini, M. M.; Peel, S. A. F.; and Davies, J. E. (1996) "Collagen Fibres are Not Required for Initial Matrix Mineralization by Bone Cells," Cells and Materials: Vol. 6 : No. 1 , Article 23. Available at: https://digitalcommons.usu.edu/cellsandmaterials/vol6/iss1/23 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Cells and Materials by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Cells and Materials Vol. 6, No. 1-3, 1996 (Pages 233-250) 1051-6794/96$5.00+ .25 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA COLLAGEN FIBRES ARE NOT REQUIRED FOR INITIAL MATRIX MINERALIZATION BY BONE CELLS M.M. Hosseini, S.A.F. Peel and J.E. Davies• Centre for Biomaterials, University of Toronto, 170 College Street, Toronto, Ontario, Canada, M5S 3E3 (Received for publication June 25, 1996 and in revised form December 27, 1996) Abstract Introduction Passaged primary cultures of young adult rat bone We have recently shown that differentiating osteo marrow cells were maintained in medium containing genic cell s, derived from explants of young adult rat combinations of the supplements dexamethasone, ascor bone marrow, elaborate an interfacial matrix with the bic acid and Na-{3-glycerophosphate. -

The Role of Bone Marrow-Derived Cells During Ectopic Bone
Int. J. Med. Sci. 2018, Vol. 15 748 Ivyspring International Publisher International Journal of Medical Sciences 2018; 15(8): 748- 757. doi: 10.7150/ijms.24605 Research Paper The Role of Bone Marrow-Derived Cells during Ectopic Bone Formation of Mouse Femoral Muscle in GFP Mouse Bone Marrow Transplantation Model Kiyofumi Takabatake1, Hidetsugu Tsujigiwa2, Yu Song1, Hiroyuki Matsuda1, Hotaka Kawai1, Masae Fujii1, Mei Hamada1, Keisuke Nakano1, Toshiyuki Kawakami3, Hitoshi Nagatsuka1 1. Department of Oral Pathology and Medicine Graduate School of Medicine, Dentistry and Pharmaceutical Science, Okayama University, Okayama, Japan; 2. Department of life science, Faculty of Science, Okayama University of Science, Okayama, Japan; 3. Hard Tissue Pathology Unit, Matsumoto Dental University Graduate School of Oral Medicine, Shiojiri, Japan. Corresponding author: Kiyofumi Takabatake, Department of Oral Pathology and Medicine, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, 2-5-1 Shikata-Cho, Okayama 700-8558, Japan. Phone: (+81) 86-2351-6651; Fax: (+81) 86-235-6654; E-mail: [email protected] ama-u.ac.jp © Ivyspring International Publisher. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY-NC) license (https://creativecommons.org/licenses/by-nc/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2017.12.27; Accepted: 2018.04.12; Published: 2018.05.22 Abstract Multipotential ability of bone marrow-derived cells has been clarified, and their involvement in repair and maintenance of various tissues has been reported. However, the role of bone marrow-derived cells in osteogenesis remains unknown. In the present study, bone marrow-derived cells during ectopic bone formation of mouse femoral muscle were traced using a GFP bone marrow transplantation model. -

Pulp Canal Obliteration After Traumatic Injuries in Permanent Teeth – Scientific Fact Or Fiction?
CRITICAL REVIEW Endodontic Therapy Pulp canal obliteration after traumatic injuries in permanent teeth – scientific fact or fiction? Juliana Vilela BASTOS(a) Abstract: Pulp canal obliteration (PCO) is a frequent finding associated (b) Maria Ilma de Souza CÔRTES with pulpal revascularization after luxation injuries of young permanent teeth. The underlying mechanisms of PCO are still unclear, (a) Universidade Federal de Minas Gerais - and no experimental scientific evidence is available, except the results UFMG, School of Dentistry, Department of Restorative Dentistry, Belo Horizonte, MG, of a single histopathological study. The lack of sound knowledge Brazil. concerning this process gives rise to controversies, including the (b) Pontifícia Universidade Católica de Minas most suitable denomination. More than a mere semantic question, Gerais – PUC-MG, Department of Dentistry, the denomination is an important issue, because it reflects the nature Belo Horizonte, MG, Brazil. of this process, and directly impacts the treatment plan decision. The hypothesis that accelerated dentin deposition is related to the loss of neural control over odontoblastic secretory activity is well accepted, but demands further supportive studies. PCO is seen radiographically as a rapid narrowing of pulp canal space, whereas common clinical features are yellow crown discoloration and a lower or non-response to sensibility tests. Late development of pulp necrosis and periapical disease are rare complications after PCO, rendering prophylactic endodontic intervention -

The Fingerprint Sourcebook
CHAPTER EMBRYOLOGY AND MORPHOLOGY OF FRICTION RIDGE SKIN Kasey Wertheim CONTENTS 3 3.1 Introduction 12 3.7 Pattern Formation 4 3.2 Embryology: Establishing 18 3.8 Genetics Uniqueness and Pattern Formation in the Friction Ridge Skin 5 3.3 Limb Development 21 3.9 Uniqueness: Developmental Noise 7 3.4 Differentiation of the Friction 22 3.10 Summary: Keys to Ridge Skin Uniqueness and Pattern Formation 8 3.5 Primary Ridge Formation 22 3.11 Reviewers 11 3.6 Secondary Ridge 24 3.12 References Formation 3–1 Embryology and Morphology of Friction Ridge Skin C H A P T E R 3 CHAPTER 3 EMBRYOLOGY AND 3.1 Introduction Friction ridge skin has unique features that persist from MORPHOLOGY OF before birth until decomposition after death. Upon contact with a surface, the unique features of friction ridge skin may leave an impression of corresponding unique details. Two FRICTION RIDGE SKIN impressions can be analyzed, compared, and evaluated, and if sufficient quality and quantity of detail is present (or Kasey Wertheim lacking) in a corresponding area of both impressions, a com- petent examiner can effect an individualization or exclusion (identify or exclude an individual). The analysis, comparison, evaluation, and verification (ACE-V) methodology, combined with the philosophy of quantitative–qualitative examinations, provide the framework for practical application of the friction ridge examination discipline. But at the heart of the disci- pline is the fundamental principle that allows for conclusive determinations: the source of the impression, friction ridge skin, is unique and persistent. Empirical data collected in the medical and forensic com- munities continues to validate the premises of uniqueness and persistence. -

RANKL/OPG Ratio Regulates Odontoclastogenesis in Damaged
www.nature.com/scientificreports OPEN RANKL/OPG ratio regulates odontoclastogenesis in damaged dental pulp Daisuke Nishida1, Atsushi Arai2, Lijuan Zhao3, Mengyu Yang3, Yuko Nakamichi3, Kanji Horibe4, Akihiro Hosoya5, Yasuhiro Kobayashi3, Nobuyuki Udagawa6* & Toshihide Mizoguchi1,6* Bone-resorbing osteoclasts are regulated by the relative ratio of the diferentiation factor, receptor activator NF-kappa B ligand (RANKL) and its decoy receptor, osteoprotegerin (OPG). Dental tissue- localized-resorbing cells called odontoclasts have regulatory factors considered as identical to those of osteoclasts; however, it is still unclear whether the RANKL/OPG ratio is a key factor for odontoclast regulation in dental pulp. Here, we showed that odontoclast regulators, macrophage colony-stimulating factor-1, RANKL, and OPG were detectable in mouse pulp of molars, but OPG was dominantly expressed. High OPG expression was expected to have a negative regulatory efect on odontoclastogenesis; however, odontoclasts were not detected in the dental pulp of OPG-defcient (KO) mice. In contrast, damage induced odontoclast-like cells were seen in wild-type pulp tissues, with their number signifcantly increased in OPG-KO mice. Relative ratio of RANKL/OPG in the damaged pulp was signifcantly higher than in undamaged control pulp. Pulp damages enhanced hypoxia inducible factor-1α and -2α, reported to increase RANKL or decrease OPG. These results reveal that the relative ratio of RANKL/OPG is signifcant to pulpal odontoclastogenesis, and that OPG expression is not required for maintenance of pulp homeostasis, but protects pulp from odontoclastogenesis caused by damages. Osteoclasts are monocyte/macrophage-derived multinucleated bone-resorbing cells 1–3. Bone tissue is continu- ously resorbed by osteoclasts, and is subsequently replenished by osteoblasts4. -

Development of the Skin and Its Derivatives
Development of the skin and its derivatives Resources: http://php.med.unsw.edu.au/embryology/ Larsen’s Human Embryology – Chapter 7 The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam – School of Medical Sciences, UNSW Wallace Wurth Building Room 234 – [email protected] Lecture overview Skin function and anatomy Skin origins Development of the overlying epidermis Development of epidermal appendages: Hair follicles Glands Nails Teeth Development of melanocytes Development of the Dermis Resources: http://php.med.unsw.edu.au/embryology/ Larsen’s Human Embryology – Chapter 7 The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam – School of Medical Sciences, UNSW Wallace Wurth Building Room 234 – [email protected] Skin Function and Anatomy Largest organ of our body Protects inner body from outside world (pathogens, water, sun) Thermoregulation Diverse: thick vs thin skin, scalp skin vs face skin, etc Consists of: - Overlying epidermis - Epidermal appendages: - Hair follicles, - Glands: sebaceous, sweat, apocrine, mammary - Nails - Teeth - Melanocytes - (Merkel Cells - Langerhans cells) - Dermis - Hypodermis Skin origins Trilaminar embryo Ectoderm (Neural crest) brain, spinal cord, eyes, peripheral nervous system epidermis of skin and associated structures, melanocytes, cranial connective tissues (dermis) Mesoderm musculo-skeletal system, limbs connective tissue of skin and organs urogenital system, heart, blood cells Endoderm epithelial linings of gastrointestinal and respiratory tracts Ectoderm -
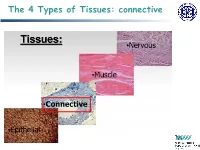
The 4 Types of Tissues: Connective
The 4 Types of Tissues: connective Connective Tissue General structure of CT cells are dispersed in a matrix matrix = a large amount of extracellular material produced by the CT cells and plays a major role in the functioning matrix component = ground substance often crisscrossed by protein fibers ground substance usually fluid, but it can also be mineralized and solid (bones) CTs = vast variety of forms, but typically 3 characteristic components: cells, large amounts of amorphous ground substance, and protein fibers. Connective Tissue GROUND SUBSTANCE In connective tissue, the ground substance is an amorphous gel-like substance surrounding the cells. In a tissue, cells are surrounded and supported by an extracellular matrix. Ground substance traditionally does not include fibers (collagen and elastic fibers), but does include all the other components of the extracellular matrix . The components of the ground substance vary depending on the tissue. Ground substance is primarily composed of water, glycosaminoglycans (most notably hyaluronan ), proteoglycans, and glycoproteins. Usually it is not visible on slides, because it is lost during the preparation process. Connective Tissue Functions of Connective Tissues Support and connect other tissues Protection (fibrous capsules and bones that protect delicate organs and, of course, the skeletal system). Transport of fluid, nutrients, waste, and chemical messengers is ensured by specialized fluid connective tissues, such as blood and lymph. Adipose cells store surplus energy in the form of fat and contribute to the thermal insulation of the body. Embryonic Connective Tissue All connective tissues derive from the mesodermal layer of the embryo . The first connective tissue to develop in the embryo is mesenchyme , the stem cell line from which all connective tissues are later derived. -

The Role of the Mesenchyme in 1. Patterns of Mesenchymal Cell
THE AMERICAN JOURNAL OF ANATOMY 188:121-132 (1990) The Role of the Mesenchyme in Mouse Neural Fold Elevation 1. Patterns of Mesenchymal Cell Distribution and Proliferation in Embryos Developing In Vitro JOYCE MORRIS-WIMAN AND LINDA L. BRINKLEY Department of Anatomy & Cell Biology, University of Michigan Medical School, Ann Arbor, Michigan 48109 ABSTRACT Using the computer-assisted a decrease in the density of cells residing in the central method of smoothed spatial averaging, spatial region of the mesenchyme and with a decrease in the and temporal patterns of cell distribution and mi- concentration of hyaluronate (HA) in this region (Mor- totic activity were analyzed in the cranial mesen- ris-Wiman and Brinkley, 1989). The decrease observed chyme underlying the mesencephalic neural folds in mesenchymal cell density during elevation could re- sult from a decrease in mitotic rate, from an increase in of mouse embryos maintained in roller tube cul- cell death, or from the expanion of the extracellular ture. Total cell density increased in central and matrix of the cranial mesenchyme displacing cells from medial mesenchymal regions after 12 hr in cul- this region. The glycosaminoglycan hyaluronate is the ture, decreased after 18 hr, and showed a further major component of this extracellular matrix (Solursh decrease after 24 hr when the neural folds of the and Morriss, 1977; Morriss and Solursh, 1978a,b;Hei- embryos had elevated, converged, and were fus- fetz et al., 1980). In biological and experimental sys- ing or fused. Mitotic activity, as measured by the tems, it is capable of a high degree of hydration with a ratio of 3H-thymidine-labeled cells to unlabeled concomitant increase in volume (Comper and Laurent, cells, was highest in the central mesenchyme at all 1978). -

Role of Extracellular Matrix in Regulating Embryonic Epithelial-Mesenchymal Transition
BioMol Concepts, Vol. 3 (2012), pp. 333–344 • Copyright © by Walter de Gruyter • Berlin • Boston. DOI 10.1515/bmc-2011-0065 Review Role of extracellular matrix in regulating embryonic epithelial-mesenchymal transition Francesca Zito considerable amount of data about its basic mechanisms. Istituto di Biomedicina e Immunologia Molecolare From a clinical viewpoint, the results obtained so far offer ‘ Alberto Monroy ’ , Consiglio Nazionale delle Ricerche, a great promise for the development of new therapies. At Via Ugo La Malfa 153, I-90146 Palermo , Italy the same time, they advance our knowledge about essential developmental processes. e-mail: [email protected] Although EMT is a widely studied topic, it is likely that not all aspects of this process have been elucidated so far. Considerable knowledge has been accumulated regarding cell Abstract morphological changes, and the regulation of several genes and various signaling pathways have been partially under- Embryogenesis and morphogenesis are characterized by stood. Still, little is known about the role of the extracellular complex cell rearrangements and movements which require matrix (ECM) as a cell substrate during EMT, and cell motility appropriate interactions of cells with the surrounding extracel- is probably the least understood aspect of the whole process. lular matrix (ECM) by means of specifi c membrane receptors. Many events in developing embryo are associated with Interest in the identifi cation and purifi cation of ECM com- cell migration, which consists of the coordinated move- ponents, as well as in conducting functional studies of them, ments of several different cells over short or long distances including their ligands and other molecules involved in cell- throughout the embryo. -

Metamorphic Changes of Dermis in Skin of Frog Larvae Exposed to Thyroxine’
DEVELOPMENTAL BIOLOGY, 7, 244-254 (1963) Metamorphic Changes of Dermis in Skin of Frog Larvae Exposed to Thyroxine’ NORMAN E. KEMP Department of Zoology, The University of Michigan, Ann Arbor, Michigan Accepted October 22, 1962 INTRODUCTION Skin in the tadpole of anuran larvae prior to metamorphosis is composed of two layers of epidermal cells (Leeson and Threadgold, 1961; Chapman and Dawson, 1961; Edds and Sweeny, 1960, 1962), a laminated basement lamella, and a layer of dermal mesenchyme cells apposed to the underside of the basement lamella. An adepidermal space and membrane (Salpeter and Singer, 1959, 1960) separate epi- dermis from the dermal basement lamella. Weiss and Ferris (1954) first observed that mesenchyme cells migrate into the basement lamella as metamorphosis begins. Invasion of the lamella has been reported to begin about Taylor-Kollros stage XI or XII (Kemp, 1961b) and to proceed until mesenchyme cells move entirely through the lamella and cause it to become detached from the adepidermal mem- brane at stages XV-XVI. Subsequently a population of cells consti- tuting the stratum spongiosum develops beneath the adepiderma1 membrane and the basement lamella becomes the deeper-lying stra- tum compacturn or “dermal lamella” of the adult dermis. New poly- merization of collagenous fibers and ground substance results in deposition of the adult basement membrane between the adepidermal membrane and the stratum spongiosum. Generalized metamorphic changes can be induced in amphibian larvae by exposing them to thyroxine or other thyroid hormones (Kollros, 1959; Pitt-Rivers and Tata, 1959). Local metamorphic changes can be induced by implanting pellets of thyroxine mixed with ‘This investigation was supported by grants from the United States Public Health Service (RG 5867-C>), the National Science Foundation (G-19447), and the Michigan Memorial-Phoenix Project (no. -

Biomechanical Analysis of Hard Tissue Responses and Injuries with Finite Element Full Human Body Model
BIOMECHANICAL ANALYSIS OF HARD TISSUE RESPONSES AND INJURIES WITH FINITE ELEMENT FULL HUMAN BODY MODEL Jay Zhijian Zhao, Gopal Narwani TK Holdings Inc. United States Paper Number 07-0354 ABSTRACT for an average adult male reported earlier [1], was one such tool for injury analyses of the thorax, This paper summarizes the development activities on abdomen and shoulder of a belted occupant. the finite element full human body model, improving However, this model was not fully biofidelic and upon last 19th ESV publication (ESV 05-0399). The thus needed to be further developed. updated Takata Human Model for an average adult male has anatomical details of skeleton and major A biofidelic full human body model requires two soft tissues in all the body parts—head, neck, essential elements: the anatomical structures and the shoulder, thorax, abdomen, pelvis, lower and upper material characterization of human. All the human extremities. The arteries and veins as well as sciatic (hard and soft) tissues of which injuries were nerves in pelvis, thigh and tibia regions were also observed in field should be modeled in the modeled. The model’s responses of all the body parts anthropometrical details and their physical material were validated against published or in-house PMHS properties should be investigated. test data of twenty tissue material tests and forty- seven pendulum, drop or sled tests under frontal, side As an applicable occupant injury assessment tool the and oblique and rear impacts. A method similar to human model was required to be fully validated for those defined in the ISO-TR9790 lateral biofidelity its biofidelity.