Heteroptera: Plataspidae): a Recent Invader to the United States
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Encounters Between Aphids and Their Predators: the Relative Frequencies of Disturbance and Consumption
Blackwell Publishing Ltd Encounters between aphids and their predators: the relative frequencies of disturbance and consumption Erik H. Nelson* & Jay A. Rosenheim Center for Population Biology and Department of Entomology, One Shields Avenue, University of California, Davis, CA 95616, USA Accepted: 3 November 2005 Key words: avoidance behavior, escape behavior, induced defense, non-consumptive interactions, non-lethal interactions, predation risk, trait-mediated interactions, Aphididae, Homoptera, Aphis gossypii, Acyrthosiphon pisum Abstract Ecologists may wish to evaluate the potential for predators to suppress prey populations through the costs of induced defensive behaviors as well as through consumption. In this paper, we measure the ratio of non-consumptive, defense-inducing encounters relative to consumptive encounters (hence- forth the ‘disturbed : consumed ratio’) for two species of aphids and propose that these disturbed : consumed ratios can help evaluate the potential for behaviorally mediated prey suppression. For the pea aphid, Acyrthosiphon pisum (Harris) (Homoptera: Aphididae), the ratio of induced disturbances to consumption events was high, 30 : 1. For the cotton aphid, Aphis gossypii (Glover) (Homoptera: Aphididae), the ratio of induced disturbances to consumption events was low, approximately 1 : 14. These results indicate that the potential for predators to suppress pea aphid populations through induction of defensive behaviors is high, whereas the potential for predators to suppress cotton aphid populations through induced behaviors is low. In measuring the disturbed : consumed ratios of two prey species, this paper makes two novel points: it highlights the variability of the disturbed : con- sumed ratio, and it offers a simple statistic to help ecologists draw connections between predator–prey behaviors and predator–prey population dynamics. -

INSECTS of MICRONESIA Heteroptera: Pentatomoidea1
INSECTS OF MICRONESIA Heteroptera: Pentatomoidea1 By HERBERT RUCKES RESEARCH ASSOCIATE, DEPARTMENT OF ENTOMOLOGY AMERICAN MUSEUM OF NATURAL HISTORY, NEW YORK EMERlTUS PROFESSOR OF BIOLOGY, CITY COLLEGE OF NEW YORK INTRODUCTION The Pentatomoidea consists of the families Plataspidae, Cydnidae, Pentatomidae, Acanthosomidae, Phloeidae, Urostylidae, Aphylidae, and Lestoniidae. In this classification I am following the terminology proposed by China and Miller (l955, Ann. Mag. Nat. Hist. XII, 8: 257-267). Of these various families, representatives of the Phloeidae, Urostylidae, Lestoniidae, and Aphylidae have not, as yet, been recorded from Micronesia. The Phloei dae, represented by two genera, are found only in Brazil. The Urostylidae, however, are native to India; China, Japan, Australia, the Philippines, and intermediate islands such as Borneo and Java. It is rather surprising that examples of this family have not been taken from Micronesia since the other pentatomoid fauna of these islands is, for the most part, derived from the nearby Australian, Asian, and adjacent insular regions where the Uro stylidae occur. Aphylidae and Lestoniidae are strictly Australian families, each represented by a single genus. The remaining families have varying representation in the Micronesian fauna. I wish to take this opportunity to express my sincere thanks to Miss Marjorie Statham, of the technical staff of the Department of Entomology of the American Museum of Natural History, for generously donating her time and ability to make the fine drawings that accompany this report. Her gratuitous services are, indeed, greatly appreciated. Thanks are also extended to Miss Setsuko Nakata, of the Bernice P. Bishop Museum staff, for editing and preparing the typescript of this article for publication. -
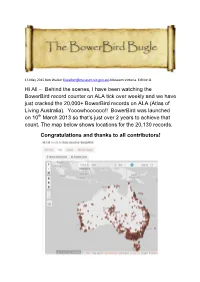
Behind the Scenes, I Have Been Watching the Bowerbird Record
15 May 2015 Ken Walker ([email protected]) Museum Victoria. Edition 8. Hi All – Behind the scenes, I have been watching the BowerBird record counter on ALA tick over weekly and we have just cracked the 20,000+ BowerBird records on ALA (Atlas of Living Australia). Yooowhoooooo!! BowerBird was launched on 10th March 2013 so that’s just over 2 years to achieve that count. The map below shows locations for the 20,130 records. Congratulations and thanks to all contributors! From a purely funding analysis point of view, the initial ALA grant to develop BowerBird was $350,000 which at 20,130 records is currently returning per record at a cost of $17.38 and that per record cost will only get lower as more BowerBird records are added and uploaded. For some species on ALA, BowerBird provides the only distributional species data points: For other species on ALA, BowerBird provides the only species image for species on ALA: From a Biosecurity point of view of tracking exotic species, BowerBird has supplied all records post 2007 for the exotic South African Carder bee. All of the blue dots represent BowerBird records. Other than the great biodiversity results that BowerBird delivers through ALA, there are a myriad of other intangible benefits that come from the BowerBird website. Intangibles such as the member’s conversations, identification discussions, the friendships, the biological statements and the generated innate knowledge. One particular “intangible”, I would like to tell you about here. The PBCRC (Plant Biosecurity, Cooperative Research Centre) has a theme called “Building Resilience through Remote Indigenous Engagement”. -

Good Water Ripples Volume 7 Number 4
For information contact: http://txmn.org/goodwater [email protected] Volume 7 Number 4 August/September 2018 Editor: Mary Ann Melton Fall Training Class Starts Soon Good Water Mas- ter Naturalist Fall Training Class will start Tuesday even- ing, September 4th. The class will meet UPCOMING EVENTS on Tuesday eve- nings from 6:00- 8/9/18 NPSOT 9:30 p.m. Some 8/13/18 WAG classes and field trips will be on Sat- 8/23/18 GWMN urdays. The first class is Tuesday, Austin Butterfly Forum 8/27/18 September 4. The 9/5/18 NPAT last class will be December 11. Cost is $150 and includes the comprehensive Texas Master 9/13/18 NPSOT Naturalist Program manual as well as a one year membership to the Good 9/20/18 Travis Audubon Water Chapter. For couples who plan to share the manual, there is a dis- count for the second student. 9/24/18 Austin Butterfly Forum Click here for online registration. The Tuesday classes will start at 6:00 9/27/18 GWMN p.m. and finish around 9:30. There are four Saturday field trips and classes planned. The schedule will be posted in the next week or so. Check back Check the website for additional here after August 15 for the link to the schedule. events including volunteer and training opportunities. The events Click here: https://txmn.org/goodwater/Training-class-online-application/ are too numerous to post here. for Online Training Registration David Robinson took our Spring Training Class this year. He says, "The Fall Training Class Starts Soon 1 Instructors & Speakers were absolutely fantastic. -

Kudzu Vine Distribution of Kudzu
Kudzu Bug Megacopta cribraria Photo: ©2007 Charles Lam, Wikimedia commons Kudzu Bug • First species of Plataspidae family in Western Hemisphere! • 2009: First detection in U.S. (Georgia) • Agricultural pest to legumes • Nuisance pest to homeowners Photos: (Top) - Michasia Harris, University of Georgia, Bugwood.org #5474627; (Bottom) - Daniel R. Suiter, University of Georgia, Bugwood.org # 5407722 Kudzu Bug Distribution No sampling Sampled but not found Intercepted or detected, but not established Established by survey or consensus Map courtesy of Pest Tracker, National Agricultural Pest Information System (NAPIS), and The University of Georgia - Center for Invasive Species and Ecosystem Health, eddmaps.org Photo: Russ Ottens, University of Georgia, Bugwood.org #5475145 Susceptible Plants In the United States: • Kudzu • Soybean and other legumes • Corn • Sweet potato • Wisteria • Wheat In native regions: • NOT an agricultural pest Kudzu bug on soybean Photo: Michasia Harris, University of Georgia, Bugwood.org #5473918 Kudzu “The vine that ate the South” Kudzu vine Distribution of Kudzu Photo: Leslie J. Mehrhoff, University of Connecticut, Bugwood.org #5483399; Map courtesy of USDA database, National Agricultural Pest Information System (NAPIS) Identification Eggs Nymphs Symbiont capsules in egg mass Hairy appearance of nymphs Photos: (Top) - Joe Eger, Dow AgroSciences, Bugwood.org #5471831; Yasmin Cardoza, North Carolina State University, Bugwood.org #5472919 (Bottom) - Yasmin Cardoza, North Carolina State University, Bugwood.org #5472911; -

Your HATCH Project Sample Title Here
HATCH PROJECT Alabama Agricultural Experiment Station Auburn University TITLE OF PROPOSED PROJECT: Development and Implementation of Alternative Pest Management Strategies for Emerging Crop Pests in Alabama PRINCIPAL INVESTIGATOR: Department: 1 SUMMARY OF CRIS DATABASE SEARCH A search of the Current Research Information System (CRIS) revealed a total of eight projects relating to arthropod pest management. Entering “insect pest management” returned 6 matches, while entering “insect pest management in fruit/vegetable/specialty crops” returned 2 matches. Four of these are Hatch projects and only two are vaguely related to the focus of this Hatch project: 1) Development and Implementation of New Reduced-Risk Insect Management Strategies for Blueberries and Cranberries - by ??, ?? University, 2) Insect Pest Management of Sweet Potato and Vegetable Crops – by :??, ?? University, and 3) High Value Specialty Crop Pest Management – by ??, ?? University. None of the projects directly addresses the specific objectives of or overlaps with this proposed Hatch project. NON TECHNICAL SUMMARY This project focuses on the management of key emerging pests of crops in Alabama, specifically pests of fruit and vegetable crops and soybean. Fruit and vegetable crops constitute an important group of horticultural crops in the U.S. with an annual market value of approximately $23 billion. Soybean is also an important crop in the U.S with an annual market value of about $42 billion. Several arthropod pests attack these crops in Alabama with the potential to cause significant economic losses to producers. The goal of this project is to develop and implement ecologically based and cost-effective integrated pest management (IPM) practices for major and emerging pests of peaches, cucurbits, crucifers and soybean. -

EPPO Reporting Service
ORGANISATION EUROPEENNE EUROPEAN AND MEDITERRANEAN ET MEDITERRANEENNE PLANT PROTECTION POUR LA PROTECTION DES PLANTES ORGANIZATION EPPO Reporting Service NO. 09 PARIS, 2014-09-01 CONTENTS _______________________________________________________________________ Pests & Diseases 2014/158 - New additions to the EPPO A1 and A2 Lists 2014/159 - PQR - the EPPO database on quarantine pests: new update 2014/160 - EPPO Global Database has just been launched (https://gd.eppo.int/) 2014/161 - Megacopta cribraria is an emerging pest of soybean in the USA: addition to the EPPO Alert List 2014/162 - Interception of Callidiellum villosulum in Malta 2014/163 - A new disease of coast live oaks (Quercus agrifolia) in California (US) is associated with Geosmithia pallida and Pseudopityophthorus pubipennis 2014/164 - First report of Globodera pallida in Bosnia and Herzegovina 2014/165 - First report of Xanthomonas fragariae in Mexico 2014/166 - Studies on olive (Olea europaea) as a host of Xylella fastidiosa in California (US) 2014/167 - First reports of ‘Candidatus Liberibacter asiaticus’ in Guadeloupe and Martinique 2014/168 - Tomato apical stunt viroid detected on tomatoes in Italy 2014/169 - Survey on Potato spindle tuber viroid and Tomato apical stunt viroid in ornamental plants in Croatia. 2014/170 - First report of Fusarium oxysporum f. sp. cubense tropical race 4 in Jordan 2014/171 - New data on quarantine pests and pests of the EPPO Alert List CONTENTS ___________________________________________________________________________ Invasive Plants 2014/172 -

Hemiptera: Miridae: Orthotylinae) in Different Instars
RESEARCH ARTICLE The structure and morphologic changes of antennae of Cyrtorhinus lividipennis (Hemiptera: Miridae: Orthotylinae) in different instars 1☯ 2☯ 3 3 1 Han-Ying Yang , Li-Xia Zheng , Zhen-Fei Zhang , Yang Zhang , Wei-Jian WuID * 1 Laboratory of Insect Ecology, South China Agricultural University, Guangzhou, China, 2 College of Agronomy, Jiangxi Agricultural University, Nanchang, China, 3 Plant Protection Institute, Guangdong a1111111111 Agricultural Science Academy, Guangzhou, China a1111111111 a1111111111 ☯ These authors contributed equally to this work. a1111111111 * [email protected] a1111111111 Abstract Cyrtorhinus lividipennis Reuter (Hemiptera: Miridae: Orthotylinae), including nymphs and OPEN ACCESS adults, are one of the dominant predators and have a significant role in the biological con- Citation: Yang H-Y, Zheng L-X, Zhang Z-F, Zhang trol of leafhoppers and planthoppers in irrigated rice. In this study, we investigated the Y, Wu W-J (2018) The structure and morphologic antennal morphology, structure and sensilla distribution of C. lividipennis in different changes of antennae of Cyrtorhinus lividipennis instars using scanning electron microscopy. The antennae of both five different nymphal (Hemiptera: Miridae: Orthotylinae) in different instars. PLoS ONE 13(11): e0207551. https://doi. stages and adults were filiform in shape, which consisted of the scape, pedicel and flagel- org/10.1371/journal.pone.0207551 lum with two flagellomeres. There were significant differences found in the types of anten- Editor: Feng ZHANG, Nanjing Agricultural nal sensilla between nymphs and adults. The multiporous placodea sensilla (MPLA), University, CHINA basiconica sensilla II (BAS II), and sensory pits (SP) only occurred on the antennae of Received: August 8, 2018 adult C. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

A Key and Annotated List of the Scutelleroidea of Michigan (Hemiptera)
The Great Lakes Entomologist Volume 3 Number 2 -- Summer 1970 Number 2 -- Summer Article 1 1970 July 2017 A Key and Annotated List of the Scutelleroidea of Michigan (Hemiptera) J.E. McPherson Southern Illinois University Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation McPherson, J.E. 2017. "A Key and Annotated List of the Scutelleroidea of Michigan (Hemiptera)," The Great Lakes Entomologist, vol 3 (2) Available at: https://scholar.valpo.edu/tgle/vol3/iss2/1 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. McPherson: A Key and Annotated List of the Scutelleroidea of Michigan (Hemip 34 THE MICHIGAN ENTOMOLOGIST Vol. 3, No. 2 A KEY AND ANNOTATED LIST OF THE SCUTELLEROIDEA OF MICHIGAN (HEMIPTERA) Department of Zoology, Southern Illinois University Carbondale, Illinois 6290 1 Although Hussey (1922) compiled a list of the Hemiptera of Berrien County, and Stoner (1922) contributed a fist of the Scutelleroidea of the Douglas Lake region, no publications have dealt with Michigan Scutelleroidea on a state-wide basis. However, collections in the Entomology Museum of Michigan State University (MSU), East Lansing, and in the Museum of Zoology of the University of Michigan (UMMZ), Ann Arbor, indicate that collecting has been extensive throughout the state (Fig. 1). The key and annotated list are based on material I identified in these two collections. -

Biology and Dispersal of the Watermelon Bug Coridius Viduatus (F.) (Heteroptera: Dinidoridae) on Different Cucurbit Crops, in North Darfur State, Sudan
Asian Research Journal of Agriculture 10(3): 1-9, 2018; Article no.ARJA.45722 ISSN: 2456-561X Biology and Dispersal of the Watermelon Bug Coridius viduatus (F.) (Heteroptera: Dinidoridae) on Different Cucurbit Crops, in North Darfur State, Sudan Amin El Zubeir Gubartalla1*, Ibrahim Abdel–Rahman Ibrahim2 and Salha Mahmoud Solum3 1Department of Plant Protection and Environmental Studies, Faculty of Agriculture, Alzaiem Alazhari University, Sudan. 2Department of Plant Protection, Faculty of Environmental Sciences and Natural Resources, University of Al-Fashir, Sudan. 3Minstry of Agriculture, Irrigation and Range-North Darfur, Sudan. Authors’ contributions This work was carried out in collaboration between all authors. All authors read and approved the final manuscript. Article Information DOI: 10.9734/ARJA/2018/45722 Editor(s): (1) Dr. Gabriel Oladele Awe, Department of Soil Resources & Environmental Management, Faculty of Agricultural Sciences, Ekiti State University, Nigeria. (2) Dr. Mahmoud Hozayn, Professor, Department of Field Crops Research, Division of Agricultural and Biological Research, National Research Centre, Cairo, Egypt. Reviewers: (1) Bonaventure January, Sokoine University of Agriculture, Tanzania. (2) Aba-Toumnou Lucie, University of Bangui, Central African Republic. Complete Peer review History: http://www.sciencedomain.org/review-history/28049 Received 17 September 2018 Accepted 06 December 2018 Original Research Article Published 01 January 2019 ABSTRACT The watermelon bug, Coridius viduatus (F.) is a real threat to watermelon Citrullus lanatus (Thunb.) in western Sudan, where over 80% of the population relies economically on agriculture. In order to overcome this constraint, a study was carried out at University of Alfashir, North Darfur State, to investigate biology, food preference and dispersal of watermelon bug. -

Chemical Analysis of the Metathoracic Scent Gland of Eurygaster Maura (L.) (Heteroptera: Scutelleridae)
J. Agr. Sci. Tech. (2019) Vol. 21(6): 1473-1484 Chemical Analysis of the Metathoracic Scent Gland of Eurygaster maura (L.) (Heteroptera: Scutelleridae) E. Ogur1, and C. Tuncer2 ABSTRACT Eurygaster maura (L.) (Heteroptera: Scutelleridae) is one of the most devastating pests of wheat in Turkey. The metathoracic scent gland secretions of male and female E. maura were analyzed separately by gas chromatography-mass spectrometry. Twelve chemical compounds, namely, Octane, n-Undecane, n-Dodecane, n-Tridecane, (E)-2-Hexenal , (E)- 2-Hexen-1-ol, acetate, Cyclopropane, 1-ethyl-2-heptyl, Hexadecane, (E)-3-Octen-1-ol, acetate, (E)-5-Decen-1-ol, acetate, 2-Hexenoic acid, Butyric acid, and Tridecyl ester were detected in both males and females. These compounds, however, differed quantitatively between the sexes. In both females and males, n-Tridecane and (E)-2-Hexanal were the most abundant compounds and constituted approximately 90% of the total content. Minimal amounts of Octane were detected in males and Hexadecane in females. Keywords: (E)-2-Hexenal, GC-MS, Metathoracic scent gland, n-Tridecane, Wheat. INTRODUCTION 100% in the absence of control measures (Lodos, 1986; Özbek and Hayat, 2003). Eurygaster species (Heteroptera: In the order Heteroptera, nearly all species Scutelleridae) are the most devastating pests have scent glands and many of these are of wheat in an extensive area of the Near colloquially referred to as “stink bugs” and Middle East, Western and Central Asia, (Aldrich, 1988). Both nymphs and adults Eastern and South Central Europe, and have scent glands in Heteroptera species Northern Africa (Critchley, 1998; Vaccino (Abad and Atalay, 1994; Abad, 2000).