The Functional Role of the Anterior Insular Cortex in Cognitive Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

Dopamine Neurons Mediate a Fast Excitatory Signal Via Their Glutamatergic Synapses
972 • The Journal of Neuroscience, January 28, 2004 • 24(4):972–981 Cellular/Molecular Dopamine Neurons Mediate a Fast Excitatory Signal via Their Glutamatergic Synapses Nao Chuhma,1,6 Hui Zhang,2 Justine Masson,1,6 Xiaoxi Zhuang,7 David Sulzer,1,2,6 Rene´ Hen,3,5 and Stephen Rayport1,4,5,6 Departments of 1Psychiatry, 2Neurology, 3Pharmacology, and 4Anatomy and Cell Biology, and 5Center for Neurobiology and Behavior, Columbia University, New York, New York 10032, 6Department of Neuroscience, New York State Psychiatric Institute, New York, New York 10032, and 7Department of Neurobiology, Pharmacology and Physiology, University of Chicago, Chicago, Illinois 60637 Dopamine neurons are thought to convey a fast, incentive salience signal, faster than can be mediated by dopamine. A resolution of this paradox may be that midbrain dopamine neurons exert fast excitatory actions. Using transgenic mice with fluorescent dopamine neurons, in which the axonal projections of the neurons are visible, we made horizontal brain slices encompassing the mesoaccumbens dopamine projection. Focal extracellular stimulation of dopamine neurons in the ventral tegmental area evoked dopamine release and early monosynaptic and late polysynaptic excitatory responses in postsynaptic nucleus accumbens neurons. Local superfusion of the ventral tegmental area with glutamate, which should activate dopamine neurons selectively, produced an increase in excitatory synaptic events. Local superfusion of the ventral tegmental area with the D2 agonist quinpirole, which should increase the threshold for dopamine neuron activation, inhibited the early response. So dopamine neurons make glutamatergic synaptic connections to accumbens neurons. We propose that dopamine neuron glutamatergic transmission may be the initial component of the incentive salience signal. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Interoception: the Sense of the Physiological Condition of the Body AD (Bud) Craig
500 Interoception: the sense of the physiological condition of the body AD (Bud) Craig Converging evidence indicates that primates have a distinct the body share. Recent findings that compel a conceptual cortical image of homeostatic afferent activity that reflects all shift resolve these issues by showing that all feelings from aspects of the physiological condition of all tissues of the body. the body are represented in a phylogenetically new system This interoceptive system, associated with autonomic motor in primates. This system has evolved from the afferent control, is distinct from the exteroceptive system (cutaneous limb of the evolutionarily ancient, hierarchical homeostatic mechanoreception and proprioception) that guides somatic system that maintains the integrity of the body. These motor activity. The primary interoceptive representation in the feelings represent a sense of the physiological condition of dorsal posterior insula engenders distinct highly resolved the entire body, redefining the category ‘interoception’. feelings from the body that include pain, temperature, itch, The present article summarizes this new view; more sensual touch, muscular and visceral sensations, vasomotor detailed reviews are available elsewhere [1,2]. activity, hunger, thirst, and ‘air hunger’. In humans, a meta- representation of the primary interoceptive activity is A homeostatic afferent pathway engendered in the right anterior insula, which seems to provide Anatomical characteristics the basis for the subjective image of the material self as a feeling Cannon [3] recognized that the neural processes (auto- (sentient) entity, that is, emotional awareness. nomic, neuroendocrine and behavioral) that maintain opti- mal physiological balance in the body, or homeostasis, must Addresses receive afferent inputs that report the condition of the Atkinson Pain Research Laboratory, Division of Neurosurgery, tissues of the body. -

The Contribution of Sensory System Functional Connectivity Reduction to Clinical Pain in Fibromyalgia
1 The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia Jesus Pujol1,2, Dídac Macià1, Alba Garcia-Fontanals3, Laura Blanco-Hinojo1,4, Marina López-Solà1,5, Susana Garcia-Blanco6, Violant Poca-Dias6, Ben J Harrison7, Oren Contreras-Rodríguez1, Jordi Monfort8, Ferran Garcia-Fructuoso6, Joan Deus1,3 1MRI Research Unit, CRC Mar, Hospital del Mar, Barcelona, Spain. 2Centro Investigación Biomédica en Red de Salud Mental, CIBERSAM G21, Barcelona, Spain. 3Department of Clinical and Health Psychology, Autonomous University of Barcelona, Spain. 4Human Pharmacology and Neurosciences, Institute of Neuropsychiatry and Addiction, Hospital del Mar Research Institute, Barcelona, Spain. 5Department of Psychology and Neuroscience. University of Colorado, Boulder, Colorado. 6Rheumatology Department, Hospital CIMA Sanitas, Barcelona, Spain. 7Melbourne Neuropsychiatry Centre, Department of Psychiatry, The University of Melbourne, Melbourne, Australia. 8Rheumatology Department, Hospital del Mar, Barcelona, Spain The submission contains: The main text file (double-spaced 39 pages) Six Figures One supplemental information file Corresponding author: Dr. Jesus Pujol Department of Magnetic Resonance, CRC-Mar, Hospital del Mar Passeig Marítim 25-29. 08003, Barcelona, Spain Email: [email protected] Telephone: + 34 93 221 21 80 Fax: + 34 93 221 21 81 Synopsis/ Summary Clinical pain in fibromyalgia is associated with functional changes at different brain levels in a pattern suggesting a general weakening of sensory integration. 2 Abstract Fibromyalgia typically presents with spontaneous body pain with no apparent cause and is considered pathophysiologically to be a functional disorder of somatosensory processing. We have investigated potential associations between the degree of self-reported clinical pain and resting-state brain functional connectivity at different levels of putative somatosensory integration. -
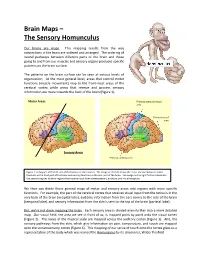
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Revista Brasileira De Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012
Rev Bras Psiquiatr. 2012;34:101-111 Revista Brasileira de Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012 REVIEW ARTICLE Neuroimaging in specific phobia disorder: a systematic review of the literature Ila M.P. Linares,1 Clarissa Trzesniak,1 Marcos Hortes N. Chagas,1 Jaime E. C. Hallak,1 Antonio E. Nardi,2 José Alexandre S. Crippa1 ¹ Department of Neuroscience and Behavior of the Ribeirão Preto Medical School, Universidade de São Paulo (FMRP-USP). INCT Translational Medicine (CNPq). São Paulo, Brazil 2 Panic & Respiration Laboratory. Institute of Psychiatry, Universidade Federal do Rio de Janeiro (UFRJ). INCT Translational Medicine (CNPq). Rio de Janeiro, Brazil Received on August 03, 2011; accepted on October 12, 2011 DESCRIPTORS Abstract Neuroimaging; Objective: Specific phobia (SP) is characterized by irrational fear associated with avoidance of Specific Phobia; specific stimuli. In recent years, neuroimaging techniques have been used in an attempt to better Review; understand the neurobiology of anxiety disorders. The objective of this study was to perform a Anxiety Disorder; systematic review of articles that used neuroimaging techniques to study SP. Method: A literature Phobia. search was conducted through electronic databases, using the keywords: imaging, neuroimaging, PET, spectroscopy, functional magnetic resonance, structural magnetic resonance, SPECT, MRI, DTI, and tractography, combined with simple phobia and specific phobia. One-hundred fifteen articles were found, of which 38 were selected for the present review. From these, 24 used fMRI, 11 used PET, 1 used SPECT, 2 used structural MRI, and none used spectroscopy. Result: The search showed that studies in this area were published recently and that the neuroanatomic substrate of SP has not yet been consolidated. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: a Central Pathway in Psychiatric Disease and Treatment
REVIEW published: 27 December 2016 doi: 10.3389/fnsys.2016.00104 Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment Sarah K. Peters 1, Katharine Dunlop 1 and Jonathan Downar 1,2,3,4* 1Institute of Medical Science, University of Toronto, Toronto, ON, Canada, 2Krembil Research Institute, University Health Network, Toronto, ON, Canada, 3Department of Psychiatry, University of Toronto, Toronto, ON, Canada, 4MRI-Guided rTMS Clinic, University Health Network, Toronto, ON, Canada The salience network (SN) plays a central role in cognitive control by integrating sensory input to guide attention, attend to motivationally salient stimuli and recruit appropriate functional brain-behavior networks to modulate behavior. Mounting evidence suggests that disturbances in SN function underlie abnormalities in cognitive control and may be a common etiology underlying many psychiatric disorders. Such functional and anatomical abnormalities have been recently apparent in studies and meta-analyses of psychiatric illness using functional magnetic resonance imaging (fMRI) and voxel- based morphometry (VBM). Of particular importance, abnormal structure and function in major cortical nodes of the SN, the dorsal anterior cingulate cortex (dACC) and anterior insula (AI), have been observed as a common neurobiological substrate across a broad spectrum of psychiatric disorders. In addition to cortical nodes of the SN, the network’s associated subcortical structures, including the dorsal striatum, mediodorsal thalamus and dopaminergic brainstem nuclei, comprise a discrete regulatory loop circuit. Edited by: The SN’s cortico-striato-thalamo-cortical loop increasingly appears to be central to Avishek Adhikari, mechanisms of cognitive control, as well as to a broad spectrum of psychiatric illnesses Stanford University, USA and their available treatments. -

Brain Responses to Different Types of Salience in Antipsychotic Naïve First Episode Psychosis: an Fmri Study
bioRxiv preprint doi: https://doi.org/10.1101/263020; this version posted September 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Brain responses to different types of salience in antipsychotic naïve first episode psychosis: An fMRI study Franziska Knolle1,3*, Anna O Ermakova2*, Azucena Justicia2,4, Paul C Fletcher2,5,6, Nico Bunzeck7, Emrah Düzel8,9, Graham K Murray2,3,5 Running title: Salience signals in antipsychotic naïve first episode psychosis *joint first author with equal contribution to the work Affiliations 1 Department of Psychiatry, University of Cambridge, Cambridge, UK 2 Unit for Social & Community Psychiatry, WHO Collaborating Centre for Mental Health Services Development, East London NHS Foundation Trust 3 Behavioural and Clinical Neuroscience Institute, University of Cambridge 4 IMIM (Hospital del Mar Medical Research Institute). Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Barcelona, Spain. 5 Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, UK 6 Institute of Metabolic Science, University of Cambridge 7 Institute of Psychology, University of Lübeck, Lübeck, Germany 8 Otto-von-Guericke University Magdeburg, Institute of Cognitive Neurology and Dementia Research, Magdeburg, Germany 9 German Centre for Neurodegenerative Diseases (DZNE), Magdeburg, Germany 1 bioRxiv preprint doi: https://doi.org/10.1101/263020; this version posted September 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Abnormal salience processing has been suggested to contribute to the formation of positive psychotic symptoms in schizophrenia and related conditions. -

Attention Or Salience?
Attention and inference Attention or salience? Thomas Parr1, Karl J Friston1 1 Wellcome Centre for Human Neuroimaging, Institute of Neurology, University College London, WC1N 3BG, UK. [email protected], [email protected] Correspondence: Thomas Parr The Wellcome Centre for Human Neuroimaging Institute of Neurology 12 Queen Square, London, UK WC1N 3BG [email protected] Abstract While attention is widely recognised as central to perception, the term is often used to mean very different things. Prominent theories of attention – notably the premotor theory – relate it to planned or executed eye movements. This contrasts with the notion of attention as a gain control process that weights the information carried by different sensory channels. We draw upon recent advances in theoretical neurobiology to argue for a distinction between attentional gain mechanisms and salience attribution. The former depends upon estimating the precision of sensory data, while the latter is a consequence of the need to actively engage with the sensorium. Having established this distinction, we consider the intimate relationship between attention and salience. Keywords: Attention; Salience; Bayesian; Active inference; Precision; Active vision Introduction Optimal interaction with the world around us requires that we attend to those sources of information that help us form accurate beliefs about states of affairs in the world (and our body). This statement may be interpreted in two very different ways. The first interpretation is that we (covertly) select from multiple sensory channels (either within or between modalities) and ascribe greater weight to those sensory streams that convey the most reliable information about states of the world [1]. -

Dopaminergic Basis of Salience Dysregulation in Psychosis
Review Dopaminergic basis of salience dysregulation in psychosis 1* 1,2* 3 Toby T. Winton-Brown , Paolo Fusar-Poli , Mark A. Ungless , and 1,3 Oliver D. Howes 1 Department of Psychosis Studies, Institute of Psychiatry, King’s College London, De Crespigny Park, SE58AF London, UK 2 OASIS Prodromal Service, South London and Maudsley (SLaM) National Health Service (NHS) Foundation Trust, London, UK 3 Medical Research Council Clinical Sciences Centre, Imperial College London, London, UK Disrupted salience processing is proposed as central in In recent years, there have been attempts to bridge this linking dysregulated dopamine function with psychotic gap. We will critically review here the evidence for the symptoms. Several strands of evidence are now converg- recent interpretation of dopaminergic dysfunction in psy- ing in support of this model. Animal studies show that chosis, according to which delusions emerge as an individ- midbrain dopamine neurons are activated by unexpected ual’s own explanation of the experience of aberrant salient events. In psychotic patients, neurochemical stud- salience. We start by examining normal aspects of salience ies have confirmed subcortical striatal dysregulation of and salience processing and how these relate to dopamine dopaminergic neurotransmission, whereas functional function in the human brain. We then describe the experi- magnetic resonance imaging (fMRI) studies of salience ence of aberrant salience in those experiencing early symp- tasks have located alterations in prefrontal and striatal toms of psychosis, before examining experimental evidence dopaminergic projection fields. At the clinical level, this of aberrant salience from animal studies and from neuro- may account for the altered sense of meaning and signifi- imaging studies in humans.