Nonlinear Schrödinger Equation and Classical-Field Description of the Lamb- Retherford Experiment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quantum Field Theory*
Quantum Field Theory y Frank Wilczek Institute for Advanced Study, School of Natural Science, Olden Lane, Princeton, NJ 08540 I discuss the general principles underlying quantum eld theory, and attempt to identify its most profound consequences. The deep est of these consequences result from the in nite number of degrees of freedom invoked to implement lo cality.Imention a few of its most striking successes, b oth achieved and prosp ective. Possible limitation s of quantum eld theory are viewed in the light of its history. I. SURVEY Quantum eld theory is the framework in which the regnant theories of the electroweak and strong interactions, which together form the Standard Mo del, are formulated. Quantum electro dynamics (QED), b esides providing a com- plete foundation for atomic physics and chemistry, has supp orted calculations of physical quantities with unparalleled precision. The exp erimentally measured value of the magnetic dip ole moment of the muon, 11 (g 2) = 233 184 600 (1680) 10 ; (1) exp: for example, should b e compared with the theoretical prediction 11 (g 2) = 233 183 478 (308) 10 : (2) theor: In quantum chromo dynamics (QCD) we cannot, for the forseeable future, aspire to to comparable accuracy.Yet QCD provides di erent, and at least equally impressive, evidence for the validity of the basic principles of quantum eld theory. Indeed, b ecause in QCD the interactions are stronger, QCD manifests a wider variety of phenomena characteristic of quantum eld theory. These include esp ecially running of the e ective coupling with distance or energy scale and the phenomenon of con nement. -

On the Definition of the Renormalization Constants in Quantum Electrodynamics
On the Definition of the Renormalization Constants in Quantum Electrodynamics Autor(en): Källén, Gunnar Objekttyp: Article Zeitschrift: Helvetica Physica Acta Band(Jahr): 25(1952) Heft IV Erstellt am: Mar 18, 2014 Persistenter Link: http://dx.doi.org/10.5169/seals-112316 Nutzungsbedingungen Mit dem Zugriff auf den vorliegenden Inhalt gelten die Nutzungsbedingungen als akzeptiert. Die angebotenen Dokumente stehen für nicht-kommerzielle Zwecke in Lehre, Forschung und für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und unter deren Einhaltung weitergegeben werden. Die Speicherung von Teilen des elektronischen Angebots auf anderen Servern ist nur mit vorheriger schriftlicher Genehmigung möglich. Die Rechte für diese und andere Nutzungsarten der Inhalte liegen beim Herausgeber bzw. beim Verlag. Ein Dienst der ETH-Bibliothek Rämistrasse 101, 8092 Zürich, Schweiz [email protected] http://retro.seals.ch On the Definition of the Renormalization Constants in Quantum Electrodynamics by Gunnar Källen.*) Swiss Federal Institute of Technology, Zürich. (14.11.1952.) Summary. A formulation of quantum electrodynamics in terms of the renor- malized Heisenberg operators and the experimental mass and charge of the electron is given. The renormalization constants are implicitly defined and ex¬ pressed as integrals over finite functions in momentum space. No discussion of the convergence of these integrals or of the existence of rigorous solutions is given. Introduction. The renormalization method in quantum electrodynamics has been investigated by many authors, and it has been proved by Dyson1) that every term in a formal expansion in powers of the coupling constant of various expressions is a finite quantity. -

THE DEVELOPMENT of the SPACE-TIME VIEW of QUANTUM ELECTRODYNAMICS∗ by Richard P
THE DEVELOPMENT OF THE SPACE-TIME VIEW OF QUANTUM ELECTRODYNAMICS∗ by Richard P. Feynman California Institute of Technology, Pasadena, California Nobel Lecture, December 11, 1965. We have a habit in writing articles published in scientific journals to make the work as finished as possible, to cover all the tracks, to not worry about the blind alleys or to describe how you had the wrong idea first, and so on. So there isn’t any place to publish, in a dignified manner, what you actually did in order to get to do the work, although, there has been in these days, some interest in this kind of thing. Since winning the prize is a personal thing, I thought I could be excused in this particular situation, if I were to talk personally about my relationship to quantum electrodynamics, rather than to discuss the subject itself in a refined and finished fashion. Furthermore, since there are three people who have won the prize in physics, if they are all going to be talking about quantum electrodynamics itself, one might become bored with the subject. So, what I would like to tell you about today are the sequence of events, really the sequence of ideas, which occurred, and by which I finally came out the other end with an unsolved problem for which I ultimately received a prize. I realize that a truly scientific paper would be of greater value, but such a paper I could publish in regular journals. So, I shall use this Nobel Lecture as an opportunity to do something of less value, but which I cannot do elsewhere. -

Feynman Quantization
3 FEYNMAN QUANTIZATION An introduction to path-integral techniques Introduction. By Richard Feynman (–), who—after a distinguished undergraduate career at MIT—had come in as a graduate student to Princeton, was deeply involved in a collaborative effort with John Wheeler (his thesis advisor) to shake the foundations of field theory. Though motivated by problems fundamental to quantum field theory, as it was then conceived, their work was entirely classical,1 and it advanced ideas so radicalas to resist all then-existing quantization techniques:2 new insight into the quantization process itself appeared to be called for. So it was that (at a beer party) Feynman asked Herbert Jehle (formerly a student of Schr¨odinger in Berlin, now a visitor at Princeton) whether he had ever encountered a quantum mechanical application of the “Principle of Least Action.” Jehle directed Feynman’s attention to an obscure paper by P. A. M. Dirac3 and to a brief passage in §32 of Dirac’s Principles of Quantum Mechanics 1 John Archibald Wheeler & Richard Phillips Feynman, “Interaction with the absorber as the mechanism of radiation,” Reviews of Modern Physics 17, 157 (1945); “Classical electrodynamics in terms of direct interparticle action,” Reviews of Modern Physics 21, 425 (1949). Those were (respectively) Part III and Part II of a projected series of papers, the other parts of which were never published. 2 See page 128 in J. Gleick, Genius: The Life & Science of Richard Feynman () for a popular account of the historical circumstances. 3 “The Lagrangian in quantum mechanics,” Physicalische Zeitschrift der Sowjetunion 3, 64 (1933). The paper is reprinted in J. -
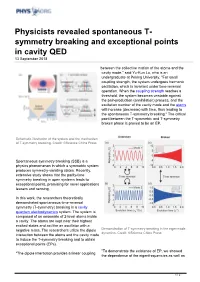
Symmetry Breaking and Exceptional Points in Cavity QED 13 September 2018
Physicists revealed spontaneous T- symmetry breaking and exceptional points in cavity QED 13 September 2018 between the collective motion of the atoms and the cavity mode," said Yu-Kun Lu, who is an undergraduate at Peking University. "For small coupling strength, the system undergoes harmonic oscillation, which is invariant under time-reversal operation. When the coupling strength reaches a threshold, the system becomes unstable against the pair-production (annihilation) process, and the excitation number of the cavity mode and the atoms will increase (decrease) with time, thus leading to the spontaneous T-symmetry breaking." The critical point between the T-symmetric and T-symmetry broken phase is proved to be an EP. Schematic illustration of the system and the mechanism of T-symmetry breaking. Credit: ©Science China Press Spontaneous symmetry breaking (SSB) is a physics phenomenon in which a symmetric system produces symmetry-violating states. Recently, extensive study shows that the parity-time symmetry breaking in open systems leads to exceptional points, promising for novel applications leasers and sensing. In this work, the researchers theoretically demonstrated spontaneous time-reversal symmetry (T-symmetry) breaking in a cavity quantum electrodynamics system. The system is composed of an ensemble of 2-level atoms inside a cavity. The atoms are kept near their highest excited states and act like an oscillator with a negative mass. The researchers utilize the dipole Demonstration of T-symmetry breaking in the eigenmode dynamics. Credit: ©Science China Press interaction between the atoms and the cavity mode to induce the T-symmetry breaking and to obtain exceptional points (EPs). "To demonstrate the existence of EP, we showed "The dipole interaction provides a linear coupling the dependence of the eigenfrequencies as well as 1 / 2 the eigenmode on the cavity-atom detuning, and we found they coalesce at the critical point, and thus proved it to be an EP," said Pai Peng, a former undergraduate in Prof. -

Structure of Vacuum in Chiral Supersymmetric Quantum Electrodynamics A
Structure of vacuum in chiral supersymmetric quantum electrodynamics A. V. Smilga Institute of Theoretical and Experimental Physics, Academy of Sciences of the USSR, Moscow (Submitted 21 January 1986) Zh. Eksp. Teor. Fiz. 91, 14-24 (July 1986) An effective Hamiltonian is found for supersymmetric quantum electrodynamics defined in a finite volume. When the right and left fields appear in the theory nonsymmetrically (but so that anomalies cancel out), this Hamiltonian turns out to be nontrivial and describes the motion of a particle in the "ionic crystal" consisting of magnetic charges of different sign with an additional scalar potential of the form U = K '/2, where aiK = z..Despite the nontrivial form of the effective Hamiltonian, supersymmetry remains unbroken. 1. INTRODUCTION analysis of nonchiral theories. We shall investigate the sim- One of the most acute problems in theoretical elemen- plest example of chiral supersymmetric QCD. Unfortunate- tary-particle physics is the question of supersymmetry ly, our original hopes have not been justified, and supersym- breaking, which is generally believed to occur in nature in metry remains unbroken in this theory. The effective one form or another at high enough energies. The most at- Hamiltonian has, however, turned out to be highly nontri- tractive mechanism of supersymmetry breaking is spontane- vial and does not reduce to free motion, as was the case for ous breaking by dynamic effects, not manifest at the "tree" nonchiral theories. The methods developed below can be level. A well-known example of this type of breaking occurs used in the analysis of more complicated chiral theories, in- in the Witten quantum mechanics with superpotential of the cluding those in which supersymmetry is definitely broken. -

Quantum Mechanics Propagator
Quantum Mechanics_propagator This article is about Quantum field theory. For plant propagation, see Plant propagation. In Quantum mechanics and quantum field theory, the propagator gives the probability amplitude for a particle to travel from one place to another in a given time, or to travel with a certain energy and momentum. In Feynman diagrams, which calculate the rate of collisions in quantum field theory, virtual particles contribute their propagator to the rate of the scattering event described by the diagram. They also can be viewed as the inverse of the wave operator appropriate to the particle, and are therefore often called Green's functions. Non-relativistic propagators In non-relativistic quantum mechanics the propagator gives the probability amplitude for a particle to travel from one spatial point at one time to another spatial point at a later time. It is the Green's function (fundamental solution) for the Schrödinger equation. This means that, if a system has Hamiltonian H, then the appropriate propagator is a function satisfying where Hx denotes the Hamiltonian written in terms of the x coordinates, δ(x)denotes the Dirac delta-function, Θ(x) is the Heaviside step function and K(x,t;x',t')is the kernel of the differential operator in question, often referred to as the propagator instead of G in this context, and henceforth in this article. This propagator can also be written as where Û(t,t' ) is the unitary time-evolution operator for the system taking states at time t to states at time t'. The quantum mechanical propagator may also be found by using a path integral, where the boundary conditions of the path integral include q(t)=x, q(t')=x' . -

Spontaneous Symmetry Breaking in the Higgs Mechanism
Spontaneous symmetry breaking in the Higgs mechanism August 2012 Abstract The Higgs mechanism is very powerful: it furnishes a description of the elec- troweak theory in the Standard Model which has a convincing experimental ver- ification. But although the Higgs mechanism had been applied successfully, the conceptual background is not clear. The Higgs mechanism is often presented as spontaneous breaking of a local gauge symmetry. But a local gauge symmetry is rooted in redundancy of description: gauge transformations connect states that cannot be physically distinguished. A gauge symmetry is therefore not a sym- metry of nature, but of our description of nature. The spontaneous breaking of such a symmetry cannot be expected to have physical e↵ects since asymmetries are not reflected in the physics. If spontaneous gauge symmetry breaking cannot have physical e↵ects, this causes conceptual problems for the Higgs mechanism, if taken to be described as spontaneous gauge symmetry breaking. In a gauge invariant theory, gauge fixing is necessary to retrieve the physics from the theory. This means that also in a theory with spontaneous gauge sym- metry breaking, a gauge should be fixed. But gauge fixing itself breaks the gauge symmetry, and thereby obscures the spontaneous breaking of the symmetry. It suggests that spontaneous gauge symmetry breaking is not part of the physics, but an unphysical artifact of the redundancy in description. However, the Higgs mechanism can be formulated in a gauge independent way, without spontaneous symmetry breaking. The same outcome as in the account with spontaneous symmetry breaking is obtained. It is concluded that even though spontaneous gauge symmetry breaking cannot have physical consequences, the Higgs mechanism is not in conceptual danger. -

Conceptual Problems in Quantum Electrodynamics: a Contemporary Historical-Philosophical Approach
Conceptual problems in quantum electrodynamics: a contemporary historical-philosophical approach (Redux version) PhD Thesis Mario Bacelar Valente Sevilla/Granada 2011 1 Conceptual problems in quantum electrodynamics: a contemporary historical-philosophical approach Dissertation submitted in fulfilment of the requirements for the Degree of Doctor by the Sevilla University Trabajo de investigación para la obtención del Grado de Doctor por la Universidad de Sevilla Mario Bacelar Valente Supervisors (Supervisores): José Ferreirós Dominguéz, Universidade de Sevilla. Henrik Zinkernagel, Universidade de Granada. 2 CONTENTS 1 Introduction 5 2 The Schrödinger equation and its interpretation Not included 3 The Dirac equation and its interpretation 8 1 Introduction 2 Before the Dirac equation: some historical remarks 3 The Dirac equation as a one-electron equation 4 The problem with the negative energy solutions 5 The field theoretical interpretation of Dirac’s equation 6 Combining results from the different views on Dirac’s equation 4 The quantization of the electromagnetic field and the vacuum state See Bacelar Valente, M. (2011). A Case for an Empirically Demonstrable Notion of the Vacuum in Quantum Electrodynamics Independent of Dynamical Fluctuations. Journal for General Philosophy of Science 42, 241–261. 5 The interaction of radiation and matter 28 1 introduction 2. Quantum electrodynamics as a perturbative approach 3 Possible problems to quantum electrodynamics: the Haag theorem and the divergence of the S-matrix series expansion 4 A note regarding the concept of vacuum in quantum electrodynamics 3 5 Conclusions 6 Aspects of renormalization in quantum electrodynamics 50 1 Introduction 2 The emergence of infinites in quantum electrodynamics 3 The submergence of infinites in quantum electrodynamics 4 Different views on renormalization 5 conclusions 7 The Feynman diagrams and virtual quanta See, Bacelar Valente, M. -

Path Integral in Quantum Field Theory Alexander Belyaev (Course Based on Lectures by Steven King) Contents
Path Integral in Quantum Field Theory Alexander Belyaev (course based on Lectures by Steven King) Contents 1 Preliminaries 5 1.1 Review of Classical Mechanics of Finite System . 5 1.2 Review of Non-Relativistic Quantum Mechanics . 7 1.3 Relativistic Quantum Mechanics . 14 1.3.1 Relativistic Conventions and Notation . 14 1.3.2 TheKlein-GordonEquation . 15 1.4 ProblemsSet1 ........................... 18 2 The Klein-Gordon Field 19 2.1 Introduction............................. 19 2.2 ClassicalScalarFieldTheory . 20 2.3 QuantumScalarFieldTheory . 28 2.4 ProblemsSet2 ........................... 35 3 Interacting Klein-Gordon Fields 37 3.1 Introduction............................. 37 3.2 PerturbationandScatteringTheory. 37 3.3 TheInteractionHamiltonian. 43 3.4 Example: K π+π− ....................... 45 S → 3.5 Wick’s Theorem, Feynman Propagator, Feynman Diagrams . .. 47 3.6 TheLSZReductionFormula. 52 3.7 ProblemsSet3 ........................... 58 4 Transition Rates and Cross-Sections 61 4.1 TransitionRates .......................... 61 4.2 TheNumberofFinalStates . 63 4.3 Lorentz Invariant Phase Space (LIPS) . 63 4.4 CrossSections............................ 64 4.5 Two-bodyScattering . 65 4.6 DecayRates............................. 66 4.7 OpticalTheorem .......................... 66 4.8 ProblemsSet4 ........................... 68 1 2 CONTENTS 5 Path Integrals in Quantum Mechanics 69 5.1 Introduction............................. 69 5.2 The Point to Point Transition Amplitude . 70 5.3 ImaginaryTime........................... 74 5.4 Transition Amplitudes With an External Driving Force . ... 77 5.5 Expectation Values of Heisenberg Position Operators . .... 81 5.6 Appendix .............................. 83 5.6.1 GaussianIntegration . 83 5.6.2 Functionals ......................... 85 5.7 ProblemsSet5 ........................... 87 6 Path Integral Quantisation of the Klein-Gordon Field 89 6.1 Introduction............................. 89 6.2 TheFeynmanPropagator(again) . 91 6.3 Green’s Functions in Free Field Theory . -

Schrödinger Equation for an Extended Electron
Schr¨odinger equation for an extended electron Antˆonio B. Nassar Physics Department The Harvard-Westlake School 3700 Coldwater Canyon, Studio City, 91604 (USA) and Department of Sciences University of California, Los Angeles, Extension Program 10995 Le Conte Avenue, Los Angeles, CA 90024 (USA) Abstract A new quantum mechanical wave equation describing the dynamics of an extended electron is derived via Bohmian mechanics. The solution to this equation is found through a wave packet approach which establishes a direct correlation between a classical variable with a quantum variable describing the dynamics of the center of mass and the width of the electron wave packet. The approach presented in this paper gives a comparatively clearer picture than approaches using elaborative manipulation of infinite series of operators. It is shown that the new Schr¨odinger equation is free of any runaway solutions or any acausal responses. PACS: 03.65.Ta, 03.65.Sq, 03.70.+k 2 About a century ago, Lorentz [1] and Abraham [2] argued that when an electron is accelerated, there are additional forces acting due to the electron’s own electromagnetic field. However, the so-called Lorentz-Abraham equation for a point-charge electron dV 2e2 d2V m = + Fext (1) dt 3c3 dt2 was found to be unsatisfactory because, for Fext = 0, it admits runaway solutions. These solutions clearly violate the law of inertia. Since the seminal works of Lorentz and Abraham, inumerous papers and textbooks have given great consideration to the proper equation of motion of an electron.[3]-[11] The problematic runaway solutions were circumvented by Sommerfeld [5] and Page [6] by going to an extended model. -

Conceptualization of the Casimir Effect
INSTITUTE OF PHYSICS PUBLISHING EUROPEAN JOURNAL OF PHYSICS Eur. J. Phys. 22 (2001) 447–451 www.iop.org/Journals/ej PII: S0143-0807(01)23822-7 Conceptualization of the Casimir effect D L Andrews andLCDavila´ Romero School of Chemical Sciences, University of East Anglia, Norwich NR4 7TJ, UK E-mail: [email protected] Received 10 April 2001 Abstract The origins and physical significance of the Casimir effect are reviewed, linking the zero-point energy of the vacuum in quantum electrodynamics with a force between conducting plates. It is shown how, by the use of dimensional and other simple physical arguments, the major features of the phenomenon can be derived. 1. Introduction Hendrik Brugt Gerhard Casimir, whose name is known throughout the physics world, died on 4 May 2000. This brief article written in his honour concerns the discovery, formulation, physical significance and impact of one of the phenomena that bears his name, the eponymous Casimir effect. The discovery of this fundamental and very general effect is remarkable in the history of science for a host of reasons, and there is an extensive literature on the subject. To quote his original publication, ‘there exists an attractive force between two metal plates which is independent of the material of the plates...’, qualified by the condition that the intervening distance is sufficiently large ‘...that for the wavelengths comparable with that distance the penetration depth is small compared with the distance’. Such an attraction between two metal plates in vacuum has nothing to do with the forces of gravity or electrostatics. ‘This force may be interpreted as a zero point pressure of electromagnetic waves’ [1].