Dynamical Gene Regulatory Networks Are Tuned by Transcriptional Autoregulation with Microrna Feedback Thomas G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systematic Comparison of Sea Urchin and Sea Star Developmental Gene Regulatory Networks Explains How Novelty Is Incorporated in Early Development
ARTICLE https://doi.org/10.1038/s41467-020-20023-4 OPEN Systematic comparison of sea urchin and sea star developmental gene regulatory networks explains how novelty is incorporated in early development Gregory A. Cary 1,3,5, Brenna S. McCauley1,4,5, Olga Zueva1, Joseph Pattinato1, William Longabaugh2 & ✉ Veronica F. Hinman 1 1234567890():,; The extensive array of morphological diversity among animal taxa represents the product of millions of years of evolution. Morphology is the output of development, therefore phenotypic evolution arises from changes to the topology of the gene regulatory networks (GRNs) that control the highly coordinated process of embryogenesis. A particular challenge in under- standing the origins of animal diversity lies in determining how GRNs incorporate novelty while preserving the overall stability of the network, and hence, embryonic viability. Here we assemble a comprehensive GRN for endomesoderm specification in the sea star from zygote through gastrulation that corresponds to the GRN for sea urchin development of equivalent territories and stages. Comparison of the GRNs identifies how novelty is incorporated in early development. We show how the GRN is resilient to the introduction of a transcription factor, pmar1, the inclusion of which leads to a switch between two stable modes of Delta-Notch signaling. Signaling pathways can function in multiple modes and we propose that GRN changes that lead to switches between modes may be a common evolutionary mechanism for changes in embryogenesis. Our data additionally proposes a model in which evolutionarily conserved network motifs, or kernels, may function throughout development to stabilize these signaling transitions. 1 Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, PA 15213, USA. -

Mothers in Science
The aim of this book is to illustrate, graphically, that it is perfectly possible to combine a successful and fulfilling career in research science with motherhood, and that there are no rules about how to do this. On each page you will find a timeline showing on one side, the career path of a research group leader in academic science, and on the other side, important events in her family life. Each contributor has also provided a brief text about their research and about how they have combined their career and family commitments. This project was funded by a Rosalind Franklin Award from the Royal Society 1 Foreword It is well known that women are under-represented in careers in These rules are part of a much wider mythology among scientists of science. In academia, considerable attention has been focused on the both genders at the PhD and post-doctoral stages in their careers. paucity of women at lecturer level, and the even more lamentable The myths bubble up from the combination of two aspects of the state of affairs at more senior levels. The academic career path has academic science environment. First, a quick look at the numbers a long apprenticeship. Typically there is an undergraduate degree, immediately shows that there are far fewer lectureship positions followed by a PhD, then some post-doctoral research contracts and than qualified candidates to fill them. Second, the mentors of early research fellowships, and then finally a more stable lectureship or career researchers are academic scientists who have successfully permanent research leader position, with promotion on up the made the transition to lectureships and beyond. -
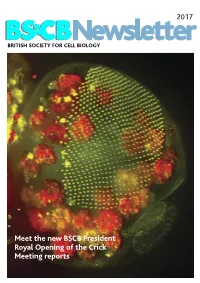
BSCB Newsletter 2017D
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

An Automatic Design Framework of Swarm Pattern Formation Based on Multi-Objective Genetic Programming
JOURNAL OF LATEX CLASS FILES, VOL. 14, NO. 8, AUGUST 2015 1 An Automatic Design Framework of Swarm Pattern Formation based on Multi-objective Genetic Programming Zhun Fan, Senior Member, IEEE, Zhaojun Wang, Xiaomin Zhu, Bingliang Hu, Anmin Zou, and Dongwei Bao Abstract—Most existing swarm pattern formation methods predetermined trajectory and maintain a specific swarm pattern depend on a predefined gene regulatory network (GRN) structure in the execution of tasks. For example, Jin [11] proposed a that requires designers’ priori knowledge, which is difficult to hierarchical gene regulatory network for adaptive multi-robot adapt to complex and changeable environments. To dynamically adapt to the complex and changeable environments, we propose pattern formation. In this work, the swarm pattern is designed an automatic design framework of swarm pattern formation as a band of circle, which encircles targets in a dynamic based on multi-objective genetic programming. The proposed environments. In the latter case of using adaptive formation, framework does not need to define the structure of the GRN- swarm robots follow an adaptive pattern to encircle targets in based model in advance, and it applies some basic network motifs the environment. For example, Oh et al. [12] have introduced to automatically structure the GRN-based model. In addition, a multi-objective genetic programming (MOGP) combines with an evolving hierarchical gene regulatory network for morpho- NSGA-II, namely MOGP-NSGA-II, to balance the complexity genetic pattern formation in order to generate adaptive patterns and accuracy of the GRN-based model. In evolutionary process, which are adaptable to dynamic environments. an MOGP-NSGA-II and differential evolution (DE) are applied to In addition, swarm pattern formation has been widely ex- optimize the structures and parameters of the GRN-based model plored in recent years, which can be divided into four cate- in parallel. -

Analysis of Murine Homeobox Genes Anthony Graham
ANALYSIS OF MURINE HOMEOBOX GENES ANTHONY GRAHAM Thesis for the Degree of Doctor of Philosophy Division of Eukaryotic Molecular Genetics National In s titu te fo r Medical Research The Ridgeway, M ill H ill London NW7 1AA Univeristy College London Gower Street London WC1E 6BT November, 1989 ProQuest Number: 10611102 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10611102 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 This thesis is dedicated to my mother and father. i i The work in this thesis is original except where stated. The sequences used for comparisons in Chapter 3 were not determined by the author but were either from publications from other laboratories or from other members of the laboratory. Anthony Graham: ABSTRACT The work in this thesis is consistent with , and strengthens, suggestions that murine homeobox genes play a role in the establishment of regional specification. It is shown that there is a correlation between the physical order of members of the Hox 2 cluster and their order of expression along the rostrocaudal axis, such that each more 3' gene is expressed more rostrally. -

Temporal Flexibility of Gene Regulatory Network Underlies a Novel Wing Pattern in Flies
Temporal flexibility of gene regulatory network underlies a novel wing pattern in flies Héloïse D. Dufoura,b, Shigeyuki Koshikawa (越川滋行)a,b,1,2,3, and Cédric Fineta,b,3,4 aHoward Hughes Medical Institute, University of Wisconsin, Madison, WI 53706; and bLaboratory of Molecular Biology, University of Wisconsin, Madison, WI 53706 Edited by Denis Duboule, University of Geneva, Geneva 4, Switzerland, and approved April 6, 2020 (received for review February 3, 2020) Organisms have evolved endless morphological, physiological, and Nevertheless, it does not explain how the new expression of behavioral novel traits during the course of evolution. Novel traits the coopted toolkit genes does not interfere with the develop- were proposed to evolve mainly by orchestration of preexisting ment of the tissue. Some authors have suggested that the reuse genes. Over the past two decades, biologists have shown that of toolkit genes might only happen during late development after cooption of gene regulatory networks (GRNs) indeed underlies completion of the early function of the redeployed genes (21, 22, numerous evolutionary novelties. However, very little is known 29). However, little is known about the properties of a GRN that about the actual GRN properties that allow such redeployment. allow the cooption of one or several of its components/genes Here we have investigated the generation and evolution of the without impairing the development of the tissue. complex wing pattern of the fly Samoaia leonensis. We show that In this study, we use the complex wing pigmentation pattern of the transcription factor Engrailed is recruited independently from the fly species Samoaia leonensis as a model to address how the the other players of the anterior–posterior specification network to generate a new wing pattern. -

Gene Regulatory Networks
Gene Regulatory Networks 02-710 Computaonal Genomics Seyoung Kim Transcrip6on Factor Binding Transcrip6on Control • Gene transcrip.on is influenced by – Transcrip.on factor binding affinity for the regulatory regions of target genes – Transcrip.on factor concentraon – Nucleosome posi.oning and chroman states – Enhancer ac.vity Gene Transcrip6onal Regulatory Network • The expression of a gene is controlled by cis and trans regulatory elements – Cis regulatory elements: DNA sequences in the regulatory region of the gene (e.g., TF binding sites) – Trans regulatory elements: RNAs and proteins that interact with the cis regulatory elements Gene Transcrip6onal Regulatory Network • Consider the following regulatory relaonships: Target gene1 Target TF gene2 Target gene3 Cis/Trans Regulatory Elements Binding site: cis Target TF regulatory element TF binding affinity gene1 can influence the TF target gene Target gene2 expression Target TF gene3 TF: trans regulatory element TF concentra6on TF can influence the target gene TF expression Gene Transcrip6onal Regulatory Network • Cis and trans regulatory elements form a complex transcrip.onal regulatory network – Each trans regulatory element (proteins/RNAs) can regulate mul.ple target genes – Cis regulatory modules (CRMs) • Mul.ple different regulators need to be recruited to ini.ate the transcrip.on of a gene • The DNA binding sites of those regulators are clustered in the regulatory region of a gene and form a CRM How Can We Learn Transcriponal Networks? • Leverage allele specific expressions – In diploid organisms, the transcript levels from the two copies of the genes may be different – RNA-seq can capture allele- specific transcript levels How Can We Learn Transcriponal Networks? • Leverage allele specific gene expressions – Teasing out cis/trans regulatory divergence between two species (WiZkopp et al. -

A Computational Model Inspired by Gene Regulatory Networks
Rui Miguel Lourenço Lopes A Computational Model Inspired by Gene Regulatory Networks Doctoral thesis submitted to the Doctoral Program in Information Science and Technology, supervised by Full Professor Doutor Ernesto Jorge Fernandes Costa, and presented to the Department of Informatics Engineering of the Faculty of Sciences and Technology of the University of Coimbra. January 2015 A Computational Model Inspired by Gene Regulatory Networks A thesis submitted to the University of Coimbra in partial fulfillment of the requirements for the Doctoral Program in Information Science and Technology by Rui Miguel Lourenço Lopes [email protected] Department of Informatics Engineering Faculty of Sciences and Technology University of Coimbra Coimbra, January 2015 Financial support by Fundação para a Ciência e a Tecnologia, through the PhD grant SFRH/BD/69106/2010. A Computational Model Inspired by Gene Regulatory Networks ©2015 Rui L. Lopes ISBN 978-989-20-5460-5 Cover image: Composition of a Santa Fe trail program evolved by ReNCoDe overlaid on the ancestors, with a 3D model of the DNA crystal structure (by Paul Hakimata). This dissertation was prepared under the supervision of Ernesto J. F. Costa Full Professor of the Department of Informatics Engineering of the Faculty of Sciences and Tecnology of the University of Coimbra In loving memory of my father. Acknowledgements Thank you very much to Prof. Ernesto Costa, for his vision on research and education, for all the support and contributions to my ideas, and for the many life stories that always lighten the mood. Thank you also to Nuno Lourenço for the many discussions, shared books, and his constant helpfulness. -

Stochasticity in the Mir-9/Hes1 Oscillatory Network Can Account For
RESEARCH ARTICLE Stochasticity in the miR-9/Hes1 oscillatory network can account for clonal heterogeneity in the timing of differentiation Nick E Phillips1, Cerys S Manning1†, Tom Pettini1†, Veronica Biga1†, Elli Marinopoulou1†, Peter Stanley1†, James Boyd1, James Bagnall1, Pawel Paszek1, David G Spiller1, Michael RH White1, Marc Goodfellow2,3,4, Tobias Galla5, Magnus Rattray1, Nancy Papalopulu1* 1Faculty of Biology, Medicine and Health, University of Manchester, Manchester, United Kingdom; 2College of Engineering, Mathematics and Physical Sciences, University of Exeter, Exeter, United Kingdom; 3Centre for Biomedical Modelling and Analysis, University of Exeter, Exeter, United Kingdom; 4EPSRC Centre for Predictive Modelling in Healthcare, University of Exeter, Exeter, United Kingdom; 5Theoretical Physics, School of Physics and Astronomy, University of Manchester, Manchester, United Kingdom Abstract Recent studies suggest that cells make stochastic choices with respect to differentiation or division. However, the molecular mechanism underlying such stochasticity is unknown. We previously proposed that the timing of vertebrate neuronal differentiation is *For correspondence: nancy. regulated by molecular oscillations of a transcriptional repressor, HES1, tuned by a post- [email protected] transcriptional repressor, miR-9. Here, we computationally model the effects of intrinsic noise on the Hes1/miR-9 oscillator as a consequence of low molecular numbers of interacting species, †These authors contributed determined experimentally. We report that increased stochasticity spreads the timing of equally to this work differentiation in a population, such that initially equivalent cells differentiate over a period of time. Competing interests: The Surprisingly, inherent stochasticity also increases the robustness of the progenitor state and lessens authors declare that no the impact of unequal, random distribution of molecules at cell division on the temporal spread of competing interests exist. -

Gene Regulatory Network Reconstruction Using Bayesian
1 Gene regulatory network reconstruction using Bayesian networks, the Dantzig selector, the Lasso and their meta-analysis Matthieu Vignes1∗, Jimmy Vandel1, David Allouche1, Nidal Ramadan-Alban1, Christine Cierco-Ayrolles1, Thomas Schiex1, Brigitte Mangin1, Simon de Givry1 1 SaAB Team/BIA Unit, INRA Toulouse, Castanet-Tolosan, France ∗ E-mail: [email protected] Abstract Modern technologies and especially next generation sequencing facilities are giving a cheaper access to genotype and genomic data measured on the same sample at once. This creates an ideal situation for multifactorial experiments designed to infer gene regulatory networks. The fifth \Dialogue for Reverse Engineering Assessments and Methods" (DREAM5) challenges are aimed at assessing methods and as- sociated algorithms devoted to the inference of biological networks. Challenge 3 on \Systems Genetics" proposed to infer causal gene regulatory networks from different genetical genomics data sets. We in- vestigated a wide panel of methods ranging from Bayesian networks to penalised linear regressions to analyse such data, and proposed a simple yet very powerful meta-analysis, which combines these inference methods. We present results of the Challenge as well as more in-depth analysis of predicted networks in terms of structure and reliability. The developed meta-analysis was ranked first among the 16 teams participating in Challenge 3A. It paves the way for future extensions of our inference method and more accurate gene network estimates in the context of genetical genomics. Introduction Inferring gene regulatory networks. Gene regulatory networks (GRN) are simplified representa- tions of mechanisms that make up the functioning of an organism under given conditions. A node in such a network stands for a gene i.e. -

MECHANISMS of DEVELOPMENT Now Continued As Cells & Development
MECHANISMS OF DEVELOPMENT Now continued as Cells & Development. AUTHOR INFORMATION PACK TABLE OF CONTENTS XXX . • Description p.1 • Audience p.1 • Impact Factor p.1 • Abstracting and Indexing p.1 • Editorial Board p.2 • Guide for Authors p.3 ISSN: 0925-4773 DESCRIPTION . Mechanisms of Development is now continued as Cells & Development. AUDIENCE . All scientists working in the fields of Cell Biology, Developmental Biology, Molecular Genetics and Differentiation. IMPACT FACTOR . 2019: 2.126 © Clarivate Analytics Journal Citation Reports 2020 ABSTRACTING AND INDEXING . EMBiology Elsevier BIOBASE Biological & Agricultural Index Biological Abstracts BIOSIS Previews Current Awareness in Biological Sciences Genetics Abstracts Science Citation Index BIOSIS Citation Index Chemical Abstracts Current Contents - Life Sciences Embase PubMed/Medline Pascal Francis Scopus AUTHOR INFORMATION PACK 3 Jan 2021 www.elsevier.com/locate/mod 1 EDITORIAL BOARD . Editor-in-Chief Roberto Mayor, University College London, London, United Kingdom Associate Editors Carl-Philipp Heisenberg, Institute of Science and Technology Austria, Klosterneuburg, Austria Sally Horne-Badovinac, University of Chicago, Chicago, Illinois, United States Ana-Maria Lennon-Duménil, Immunity and Cancer, Paris, France Guillaume Salbreux, University of Geneva, Geneva, Switzerland Didier Stainier, Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany Editorial Board Shinichi Aizawa, RIKEN Center for Developmental Biology, Kobe, Japan Miguel Allende, University of Chile Faculty -

Quantitative Single-Cell Live Imaging Links HES5 Dynamics with Cell-State and Fate in Murine Neurogenesis
ARTICLE https://doi.org/10.1038/s41467-019-10734-8 OPEN Quantitative single-cell live imaging links HES5 dynamics with cell-state and fate in murine neurogenesis Cerys S. Manning 1,7, Veronica Biga1,6, James Boyd 2,6, Jochen Kursawe1,6, Bodvar Ymisson1, David G. Spiller3, Christopher M. Sanderson2, Tobias Galla4, Magnus Rattray 5 & Nancy Papalopulu1,7 1234567890():,; During embryogenesis cells make fate decisions within complex tissue environments. The levels and dynamics of transcription factor expression regulate these decisions. Here, we use single cell live imaging of an endogenous HES5 reporter and absolute protein quantifi- cation to gain a dynamic view of neurogenesis in the embryonic mammalian spinal cord. We report that dividing neural progenitors show both aperiodic and periodic HES5 protein fluctuations. Mathematical modelling suggests that in progenitor cells the HES5 oscillator operates close to its bifurcation boundary where stochastic conversions between dynamics are possible. HES5 expression becomes more frequently periodic as cells transition to dif- ferentiation which, coupled with an overall decline in HES5 expression, creates a transient period of oscillations with higher fold expression change. This increases the decoding capacity of HES5 oscillations and correlates with interneuron versus motor neuron cell fate. Thus, HES5 undergoes complex changes in gene expression dynamics as cells differentiate. 1 School of Medical Sciences, Division of Developmental Biology and Medicine, Faculty of Biology Medicine and Health, The University of Manchester, Oxford Road, Manchester M13 9PT, UK. 2 Department of Cellular and Molecular Physiology, University of Liverpool, Crown Street, Liverpool L69 3BX, UK. 3 School of Biological Sciences, Faculty of Biology Medicine and Health, The University of Manchester, Oxford Road, Manchester M13 9PT, UK.