Management of Diabetes Before and After Surgery Or Procedure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Diabetes Mellitus Standards of Care and Clinical Practice Guidelines
WHO-EM/DIN6/E/G MANAGEMENT OF DIABETES MELLITUS STANDARDS OF CARE AND CLINICAL PRACTICE GUIDELINES Edited by Dr A.A.S. Alwan Regional Adviser, Noncommunicable Diseases WHO Regional Office for the Eastern Mediterranean WHO-EM/DIN6/E/G INTRODUCTION Available data from many countries of the Eastern Mediterranean Region (EMR) indicate that diabetes mellitus has become a problem of great magnitude and a major public health concern. Studies have demonstrated that, in some countries, diabetes affects up to 10% of the population aged 20 years and older. This rate may be doubled if those with impaired glucose tolerance (IGT) are also included. The manifestations of diabetes cause considerable human suffering and enormous economic costs. Both acute and late diabetic complications are commonly encountered. Long-term complications represented by cardiovascular diseases, cerebrovascular accidents, end-stage renal disease, retinopathy and neuropathies are already major causes of morbidity, disability and premature death in countries of this Region. The development of long-term complications is influenced by hyperglycaernia. Poor control of diabetes accelerates their progression. Thus, to prevent complications, good control of diabetes is essential and the management of diabetes should therefore aim to improve glycaemic control beyond that required to control its symptoms. Intensified therapy and maintaining near-normal blood glucose levels can result in considerable reduction in the risk of development of retinopathy, nephropathy and neuropathy. However, despite the high prevalence of diabetes and its complications and the availability of successful prevention strategies, essential health care requirements and facilities for self-care are often inadequate in this Region. Action is needed at all levels of health care and in the various aspects of diabetes care to bridge this gap and to improve health care delivery to people with diabetes. -

Environmental Risk Management for Pharmaceutical Compounds
RISK MANAGEMENT AND REGULATORY ASPECTS Environmental risk managementfor pharmaceutical compounds Nick Voulvoulis1 imperial College London Introduction Pharmaceuticals are a highly variable group of organic compounds with the potential to cause harm to aquatic ecosystems and human health. Thousands of tones of pharmacologically active substances are used annually but surprisingly little is known about their ultimate fate in the environment (Jones et al., 2001). The data collected to date, rarely provide information on the processes that determine their environmental fate and although they receive considerable pharmacological and clinical testing during development, knowledge of their ecotoxicity is poor. One major concern is that antibiotics found in sewage effluent may cause increased resistance amongst natural bacterial populations (Willis, 2000). The debate over risks associated with chemicals in the environment represents more than just another disagreement in the scientific community. It has opened the door to a new way of thinking about the onset of uninherited diseases, the nature of scientific investigation, and the role of scientific knowledge in the policymaking process. Forexample, research evidence on endocrine disruption collected over the last few years has changed dramatically the way we think about chemical risks. In part, this change has also been attributed to the precautionary principle, as a new approach to environmental policy forged in Europe. The term "precautionary approach" declares an obligation to control the dangerous substances even before a definitive causal link had been established between the chemicals and health or environmental effects, and represents a radical departure from traditional approaches to risk assessment and particularly risk management, which includes an integration of the assessment, communication and mitigation of risks. -

Optum Essential Health Benefits Enhanced Formulary PDL January
PENICILLINS ketorolac tromethamineQL GENERIC mefenamic acid amoxicillin/clavulanate potassium nabumetone amoxicillin/clavulanate potassium ER naproxen January 2016 ampicillin naproxen sodium ampicillin sodium naproxen sodium CR ESSENTIAL HEALTH BENEFITS ampicillin-sulbactam naproxen sodium ER ENHANCED PREFERRED DRUG LIST nafcillin sodium naproxen DR The Optum Preferred Drug List is a guide identifying oxacillin sodium oxaprozin preferred brand-name medicines within select penicillin G potassium piroxicam therapeutic categories. The Preferred Drug List may piperacillin sodium/ tazobactam sulindac not include all drugs covered by your prescription sodium tolmetin sodium drug benefit. Generic medicines are available within many of the therapeutic categories listed, in addition piperacillin sodium/tazobactam Fenoprofen Calcium sodium to categories not listed, and should be considered Meclofenamate Sodium piperacillin/tazobactam as the first line of prescribing. Tolmetin Sodium Amoxicillin/Clavulanate Potassium LOW COST GENERIC PREFERRED For benefit coverage or restrictions please check indomethacin your benefit plan document(s). This listing is revised Augmentin meloxicam periodically as new drugs and new prescribing LOW COST GENERIC naproxen kit information becomes available. It is recommended amoxicillin that you bring this list of medications when you or a dicloxacillin sodium CARDIOVASCULAR covered family member sees a physician or other penicillin v potassium ACE-INHIBITORS healthcare provider. GENERIC QUINOLONES captopril ANTI-INFECTIVES -

Clinical Use of Hemoglobin A1c to Improve Diabetes Management
PRACTICAL POINTERS Clinical Use of Hemoglobin A1c to Improve Diabetes Management Alan M. Delamater, PhD, ABPP or more than 25 years, the hemo- one recent study conducted in Norway6 A1C values. Only 14% of the youths globin A1c (A1C) test has been revealed that the majority (82.6%) of were able to accurately describe the A1C Fthe most widely accepted out- 201 adult patients with type 1 diabetes test. Just 11, 7.8, and 7.8% correctly come measure for evaluating glycemic knew what their last A1C was, and most identified the A1C ranges for good, fair, control in individuals with diabetes. patients (90%) knew what a satisfactory and poor glycemic control, respectively. The test provides an index of a patient’s A1C value would be. But a significant Very few youths (1.6–3.2%) knew the average blood glucose level during the number of patients (42%) reported they blood glucose values corresponding to past 2–3 months1 and is considered to had low knowledge of A1C testing in specific A1C results. Only a small num- be the most objective and reliable general. Furthermore, 25% of patients ber of youths correctly estimated the measure of long-term metabolic con- did not think that treatment intensifica- short- and long-term risks associated trol.2,3 The Diabetes Control and tion should occur at an A1C value of with A1C values of 7 and 12%. In this Complications Trial established that 10%. sample, there was a significant lack of maintaining A1C levels as close as pos- A recent cross-sectional study knowledge concerning the meaning and sible to the normal range results in con- examined the relationship between implications of the A1C test. -

The Genetics of Adverse Drug Outcomes in Type 2 Diabetes: a Systematic Review
SYSTEMATIC REVIEW published: 14 June 2021 doi: 10.3389/fgene.2021.675053 The Genetics of Adverse Drug Outcomes in Type 2 Diabetes: A Systematic Review Assefa M. Baye 1, Teferi G. Fanta 1, Moneeza K. Siddiqui 2 and Adem Y. Dawed 2* 1 Department of Pharmacology and Clinical Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia, 2 Division of Population Health and Genomics, Ninewells Hospital and School of Medicine, University of Dundee, Dundee, United Kingdom Background: Adverse drug reactions (ADR) are a major clinical problem accounting for significant hospital admission rates, morbidity, mortality, and health care costs. One-third of people with diabetes experience at least one ADR. However, there is notable interindividual heterogeneity resulting in patient harm and unnecessary medical costs. Genomics is at the forefront of research to understand interindividual variability, and there are many genotype-drug response associations in diabetes with inconsistent findings. Here, we conducted a systematic review to comprehensively examine and synthesize the effect of genetic polymorphisms on the incidence of ADRs of oral glucose-lowering drugs in people with type 2 diabetes. Edited by: Celine Verstuyft, Methods: A literature search was made to identify articles that included specific Université Paris-Saclay, France results of research on genetic polymorphism and adverse effects associated with Reviewed by: oral glucose-lowering drugs. The electronic search was carried out on 3rd October Zhiguo Xie, 2020, through Cochrane Library, PubMed, and Web of Science using keywords and Central South University, China Vera Ribeiro, MeSH terms. University of Algarve, Portugal Result: Eighteen articles consisting of 10, 383 subjects were included in this review. -

19Th Expert Committee on the Selection and Use of Essential Medicines
Expert Peer Review 2 19th Expert Committee on The Selection and Use of Essential Medicines April 8‐12 2013 Expert peer review on glibenclamide vs gliclazide, glipizide and glimepiride in the elderly. 1. Assessment of efficacy a. Have all relevant studies on efficacy been included No 1. Bollen S et al .Comparative efficacy and safety of oral diabetic medications for patients with Type 2 Diabetes AHRQ Publication No07 ‐ EHC010‐ EF July 2007 Comparative effectiveness review No. 8 A metanalysis done by these reviewers showed that there was no difference in the efficacy of Glibenclamide in reducing HbA1c as compared to Gliclazide, Glipizide and Glimepiride 2. Harrower AD. Comparison of diabetic control in type 2 (non‐insulin dependent) diabetic patients treated with different sulphonylureas. Curr Med Res Opin. 1985;9(10):676‐80. In this unblinded trial, Gliclazide was significantly better in reducing HbA1c than Glipizide 3. Schernthaner G, Grimaldi A, Di Mario U, Drzewoski J, Kempler P, Kvapil M, Novials A, Rottiers R, Rutten GE, Shaw KM.GUIDE study: double‐blind comparison of once‐daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest. 2004 Aug;34(8):535‐42. In this study once daily sustained release Gliclazide was shown to be as effective as Glimepiride in reducing HbA1c. b. Summarize the data on efficacy, in comparison to what is listed in EML where applicable (limit to 2 to 3 sentences) On metanalysis, Glibenclamide was not superior to Gliclazide, Glipizide and Glimepiride in reducing HbA1c. Gliclazide was significantly better in reducing HbA1c than Glipizide in 1 unblinded trial. -

Enhancement of Dissolution and Oral Bioavailability of Gliquidone with Hydroxy Propyl-Β-Cyclodextrin
ORIGINAL ARTICLES Pharmacology Division, Indian Institute of Chemical Technology, Hyderabad, India Enhancement of dissolution and oral bioavailability of gliquidone with hydroxy propyl-b-cyclodextrin S. Sridevi, A. S. Chauhan, K. B. Chalasani, A. K. Jain, P. V. Diwan Received November 15, 2002, accepted May 5, 2003 Dr. Prakash V. Diwan, Pharmacology Division, Indian Institute of Chemical Technology, Hyderabad – 500 007 A.P., India [email protected] Pharmazie 58: 807–810 (2003) The virtual insolubility of gliquidone in water results in poor wettability and dissolution characteristics, which may lead to a variation in bioavailability. To improve these characteristics of gliquidone, binary systems with hydroxypropyl-b-cyclodextrin (HP-b-CD) were prepared by classical methods such as physical mixing, kneading, co-evaporation and co-lyophilization. The solid state interaction between the drug and HP-b-CD was assessed by evaluating the binary systems with X-ray diffraction, differen- tial scanning calorimetry and IR- spectroscopy. The results establish the molecular encapsulation and amorphization of gliquidone. The phase solubility profile of gliquidone in aqueous HP-b-CD vehicle À1 resulted in an AL type curve with a stability constant of 1625 M . The dissolution rate of binary sys- tems was greater than that of pure drug and was significantly higher in the case of co-lyophilized and co-evaporated systems. Upon oral administration, [AUC]0-a was significantly higher in case of co-lyo- philized (2 times) and co-evaporated systems (1.5 times) compared to pure drug suspension while other binary systems showed only a marginal improvement. The study ascertained the utility of HP-b- CD in enhancing the oral bioavailability of gliquidone, and points towards a strong influence of the preparation method on the physicochemical properties. -

Medication Choice Diabetes
Weight Change Low Blood Sugar Blood Sugar Considerations (Hypoglycemia) Blood(A1c Reduction) Sugar Weight Change Low Blood Sugar (A1c Reduction) Considerations (Hypoglycemia) Metformin Metformin Metformin 1 – 2% Metformin In the rst few weeks after starting Metformin, patients may have some nausea, indigestion or diarrhea. None No Severe Risk Minor = 0 – 1% Insulin There are no other side effects associated with Insulin. Insulin Insulin Insulin Unlimited % Pioglitazone 4 to 6 lb. gain Over time, 10 in 100 people may have fluid retention Severe = 1 – 3% Minor = 30 – 40% (edema) while taking the drug. For some it may be as little as ankle swelling. For others, fluid may build up Pioglitazone 1% in the lungs making it difficult to breathe. This may Pioglitazone Pioglitazone resolve after you stop taking the drug. 10 in 100 people at risk of bone fractures who use this drug will have More than 2 to 6 lb. gain a bone fracture in the next 10 years. There appears to No Severe Risk Minor = 1 – 2% be a slight increase in the risk of bladder cancer with Liraglutide/ 0.5 – 1% this drug. Liraglutide/Exenatide Liraglutide/Exenatide Exenatide Liraglutide/Exenatide Some patients may have nausea or diarrhea. In some 3 to 6 lb. loss cases, the nausea may be severe enough that a patient No Severe Risk Minor = 0 – 1% has to stop taking the drug. There are reports of pain in the abdomen that may be caused by inammation Sulfonylureas Sulfonylureas Sulfonylureas 1 – 2% of the pancreas with these agents. Glipizide, Glimepiride, Glyburide Glipizide, Glimepiride, Glyburide Glipizide, Glimepiride, Glyburide Sulfonylureas 2 to 3 lb. -

Sulfonylurea Review
Human Journals Review Article February 2018 Vol.:11, Issue:3 © All rights are reserved by Farah Yousef et al. Sulfonylurea Review Keywords: Type II diabetes, Sulfonylurea, Glimipiride, Glybu- ride, Structure Activity Relationship. ABSTRACT Farah Yousef*1, Oussama Mansour2, Jehad Herbali3 Diabetes Mellitus is a chronic disease represented with high 1 Ph.D. candidate in pharmaceutical sciences, Damas- glucose blood levels. Although sulfonylurea compounds are the cus University, Damascus, Syria. second preferred drug to treat Type II Diabetes (TYIID), they are still the most used agents due to their lower cost and as a 2 Assistant Professor in pharmaceutical chimestry, Ti- mono-dosing. Literature divides these compounds according to st nd rd shreen University, Lattakia, Syria their discovery into 1 , 2 , 3 generations. However, only six sulfonylurea compounds are now available for use in the United 3 Assistant Professor in pharmaceutical chimestry, Da- States: Chlorpropamide, Glimepiride, Glipizide, Glyburide, mascus University, Damascus, Syria. Tolazamide, and Tolbutamide. They function by increasing Submission: 24 January 2018 insulin secretion from pancreatic beta cells. Their main active site is ATP sensitive potassium ion channels; Kir 6.2\SUR1; Accepted: 29 January 2018 Published: 28 February 2018 Potassium Inward Rectifier ion channel 6.2\ Sulfonylurea re- ceptor 1. They are sulfonamide derivatives. However, research- ers have declared that sulfonylurea moiety is not the only one responsible for this group efficacy. It has been known that sud- den and acute hypoglycemia incidences and weight gain are the www.ijppr.humanjournals.com two most common adverse effects TYIID the patient might face during treatment with sulfonylurea agents. This review indi- cates the historical development of sulfonylurea and the differ- ences among this group members. -

Association Between Serious Hypoglycemia and Calcium-Channel Blockers Used Concomitantly with Insulin Secretagogues
Research Letter | Diabetes and Endocrinology Association Between Serious Hypoglycemia and Calcium-Channel Blockers Used Concomitantly With Insulin Secretagogues Young Hee Nam, PhD; Colleen M. Brensinger, MS; Warren B. Bilker, PhD; James H. Flory, MD; Charles E. Leonard, MSCE, PharmD; Sean Hennessy, PhD, PharmD Introduction + Supplemental content Serious hypoglycemia is a major, potentially fatal adverse event caused by insulin secretagogues.1 Author affiliations and article information are Previous case reports suggested that calcium-channel blockers (CCBs) might reduce the risk of listed at the end of this article. serious hypoglycemia in patients with hyperinsulinemic hypoglycemia.2,3 However, the association of serious hypoglycemia and CCBs used with insulin secretagogues has remained unclear. Because insulin secretion by the pancreas is mediated by calcium influx in beta cells through calcium channels,4 we conducted a population-based observational study on the hypothesis that concomitant use of CCBs may be associated with reduced rates of serious hypoglycemia in insulin secretagogue users. Methods This self-controlled case series study was approved by the institutional review board of the University of Pennsylvania, which waived the requirement for informed consent because the use or disclosure of the protected health information involved no more than minimal risk to the privacy of individuals, and the research could not practicably be conducted without the waiver or alteration and without access to and use of the protected health information. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. We used claims data from the Medicaid programs of 5 US states (California, Florida, New York, Ohio, and Pennsylvania, encompassing more than a third of the nationwide Medicaid population), supplemented with Medicare claims for dual enrollees, from January 1, 1999, to December 31, 2011, and used the self- controlled case series design. -
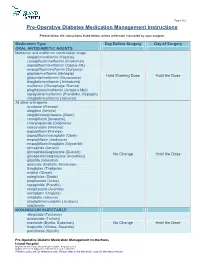
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

Glossary of Technical Terms
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. GLOSSARY OF TECHNICAL TERMS This glossary contains explanations of certain technical terms used in this Document in connection with our Company and its business. Such terminology and meanings may not correspond to standard industry meanings or usages of those terms. “acetaminophen” a non-opioid analgesic and antipyretic agent used to treat pain and fever “artificial pancreas” an integrated diabetes management system that tracks blood glucose levels using a continuous glucose monitor and automatically delivers the insulin when needed using an insulin pump according to its control algorithm “ascorbic acid” a potent reducing and antioxidant agent that functions in fighting bacterial infections, in detoxifying reactions, and in the formation of collagen in fibrous tissue, teeth, bones, connective tissue, skin, and capillaries “basal insulin” a small, continuous infusion of background insulin delivered automatically at a programmed rate, all day and night “BG Port” blood glucose strip port, the port that accepts and electrically connects a disposable blood glucose strip to the electronics “BGMS” blood glucose monitoring system “BLE” bluetooth low energy “blood glucose” blood glucose, also referred to as blood sugar, is the amount of glucose in your blood, an indicator of diabetes monitoring “bolus insulin” insulin that is taken to lower abnormally high blood glucose