Colorectal Cancer Screening and Surveillance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Colorectal Cancer Screening
CLINICAL MEDICAL POLICY Policy Name: Colorectal Cancer Screening Policy Number: MP-059-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 03/19/2021 Issue Date: 03/19/2021 Effective Date: 04/19/2021 Next Annual Review: 02/2022 Revision Date: 02/17/2021 Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 10 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ may provide coverage under the medical-surgical benefits of the Company’s Medicaid products for medically necessary colorectal cancer screening procedures. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-059-MD-PA Page 1 of 10 DEFINITIONS Average-Risk Population – Patient population defined as having no personal history of adenomatous polyps, colorectal cancer, or inflammatory bowel disease (Crohn’s disease and Ulcerative Colitis); no family history of colorectal cancer or adenomatous polyps, familial adenomatous polyposis, or hereditary nonpolyposis colorectal cancer. High-Risk Population – Patient population defined as having a first-degree relative (sibling, parent, or child) who has had colorectal cancer or adenomatous polyps; OR family history of familial adenomatous polyposis; OR family history of hereditary non-polyposis colorectal cancer; OR family history of MYH- associated polyposis in siblings; OR diagnosis of Cowden syndrome. -

Geisinger Lewistown Hospital Published: March 25, 2019
Geisinger Lewistown Hospital Published: March 25, 2019 DESCRIPTION CHARGE Fine needle aspiration; without imaging guidance $ 607.00 Fine needle aspiration; without imaging guidance $ 286.00 Fine needle aspiration; with imaging guidance $ 2,218.00 Fine needle aspiration; with imaging guidance $ 1,691.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; first lesion $ 1,979.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; each $ 1,385.00 additional lesion (List separately in addition to code for primary procedure) Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); simple or single $ 657.00 Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); complicated or $ 986.00 multiple Incision and drainage of pilonidal cyst; simple $ 657.00 Incision and drainage of pilonidal cyst; complicated $ 3,726.00 Incision and removal of foreign body, subcutaneous tissues; simple $ 1,694.00 Incision and removal of foreign body, subcutaneous tissues; complicated $ 4,710.00 Incision and drainage of hematoma, seroma or fluid collection $ 3,470.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 1,272.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 657.00 Incision -

Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications
Journal of Clinical Medicine Review Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications Francesco Restelli 1, Bianca Pollo 2 , Ignazio Gaspare Vetrano 1 , Samuele Cabras 1, Morgan Broggi 1 , Marco Schiariti 1, Jacopo Falco 1, Camilla de Laurentis 1, Gabriella Raccuia 1, Paolo Ferroli 1 and Francesco Acerbi 1,* 1 Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] (F.R.); [email protected] (I.G.V.); [email protected] (S.C.); [email protected] (M.B.); [email protected] (M.S.); [email protected] (J.F.); [email protected] (C.d.L.); [email protected] (G.R.); [email protected] (P.F.) 2 Neuropathology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-022-3932-309 Abstract: Achievement of complete resections is of utmost importance in brain tumor surgery, due to the established correlation among extent of resection and postoperative survival. Various tools have recently been included in current clinical practice aiming to more complete resections, such as neuronavigation and fluorescent-aided techniques, histopathological analysis still remains the gold-standard for diagnosis, with frozen section as the most used, rapid and precise intraoperative histopathological method that permits an intraoperative differential diagnosis. Unfortunately, due Citation: Restelli, F.; Pollo, B.; to the various limitations linked to this technique, it is still unsatisfactorily for obtaining real-time Vetrano, I.G.; Cabras, S.; Broggi, M.; intraoperative diagnosis. -

Diagnostic and Therapeutic Endoscopy of Biliary Diseases
DISEASES OF THE BILIARY TRACT, SERIES #6 Rad Agrawal, M.D., Series Editor Diagnostic and Therapeutic Endoscopy of Biliary Diseases by Yamini Subbiah, Shyam Thakkar, Elie Aoun The therapeutic approach to biliary diseases has undergone a paradigm shift over the past decade toward minimally invasive endoscopic interventions. This paper reviews the advances and different diagnostic and therapeutic endoscopic approaches to common biliary diseases including choledocholithiasis, benign and malignant biliary strictures and bile leaks. INTRODUCTION endoscopist to differentiate between benign and malig- ith the introduction of innovative endoscopic nant features, thus guiding decision making in real time. implements and options allowing for unprece- Wdented access to the biliary tree, the therapeutic COMMON BILE DUCT STONES approach to biliary diseases has undergone a significant Over 98% of biliary disorders are linked to gallstones. paradigm shift over the past decade toward minimally Stones are found in the common bile duct (CBD) in up invasive endoscopic interventions. The days where bil- to 18% of patients with symptomatic cholelithiasis (1). iary diseases were exclusively managed surgically are The vast majority of gallstones are cholesterol-rich, long gone, and much has changed since the first form in the gallbladder and gain access to the CBD via reported biliary sphincterotomies in 1974. The recent the cystic duct. De novo CBD stone formation is also developments in peroral cholangioscopy and new well described and is more common in patients of modalities of anchoring high resolution nasogastric Asian descent. These primary duct stones typically scopes in the bile duct offer the opportunity of direct have a higher bilirubin and a lower cholesterol content visualization of the bile duct lumen, which allows for and biliary stasis; further, bacterial infections have not only better identification of the underlying disease been implicated in their pathogenesis (2,3). -

Confocal Laser Endomicroscopy Platform Large Addressable Market: Early Cancer Diagnosis in GI, Urology, Interventional 2 Pulmonology, Others
Mauna Kea Technologies Corporate Presentation - November 2018 Disclaimer • This document has been prepared by Mauna Kea Technologies (the "Company") and is provided for information purposes only. • The information and opinions contained in this document speak only as of the date of this document and may be updated, supplemented, revised, verified or amended, and such information may be subject to significant changes. Mauna Kea Technologies is not under any obligation to update the information contained herein and any opinion expressed in this document is subject to change without prior notice. • The information contained in this document has not been independently verified. No representation, warranty or undertaking, express or implied, is made as to the accuracy, completeness or appropriateness of the information and opinions contained in this document. The Company, its subsidiary, its advisors and representatives accept no responsibility for and shall not be held liable for any loss or damage that may arise from the use of this document or the information or opinions contained herein. • This document contains information on the Company’s markets and competitive position, and more specifically, on the size of its markets. This information has been drawn from various sources or from the Company’s own estimates. Investors should not base their investment decision on this information. • This document contains certain forward-looking statements. These statements are not guarantees of the Company's future performance. These forward- looking statements relate to the Company's future prospects, developments and marketing strategy and are based on analyses of earnings forecasts and estimates of amounts not yet determinable. Forward-looking statements are subject to a variety of risks and uncertainties as they relate to future events and are dependent on circumstances that may or may not materialize in the future. -
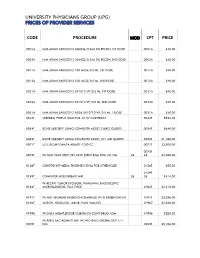
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Cycloid Scanning for Wide Field Optical Coherence Tomography Endomicroscopy and Angiography in Vivo
Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Liang, Kaicheng et al. "Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo." Optica 5, 1 (January 2018): 36-43 © 2018 Optical Society of America As Published http://dx.doi.org/10.1364/OPTICA.5.000036 Publisher OSA Publishing Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/121433 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike Detailed Terms http://creativecommons.org/licenses/by-nc-sa/4.0/ HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Optica. Manuscript Author Author manuscript; Manuscript Author available in PMC 2018 April 20. Published in final edited form as: Optica. 2018 January 20; 5(1): 36–43. doi:10.1364/OPTICA.5.000036. Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo Kaicheng Liang1,†, Zhao Wang1,†, Osman O. Ahsen1, Hsiang-Chieh Lee1, Benjamin M. Potsaid1,2, Vijaysekhar Jayaraman3, Alex Cable2, Hiroshi Mashimo4,5, Xingde Li6, and James G. Fujimoto1,* 1Department of Electrical Engineering and Computer Science, Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA 2Thorlabs, Newton, New Jersey 07860, USA 3Praevium Research, Santa Barbara, California 93111, USA 4Veterans Affairs Boston Healthcare System, Boston, Massachusetts 02130, USA 5Harvard Medical School, Boston, Massachusetts 02115, USA 6Department of Biomedical Engineering, Johns Hopkins University, Baltimore, Maryland 21218, USA Abstract Devices that perform wide field-of-view (FOV) precision optical scanning are important for endoscopic assessment and diagnosis of luminal organ disease such as in gastroenterology. -

Fees -As of 6 12 2019.Xls
• Timed charges: Some charges such as anesthesia are based on a units of time, so the charges may vary based on the units charged. • Drugs and implants: Drugs, implants and supplies are priced individually based on the cost loaded into our information systems at the time of charging, so they do not have individual prices listed. The typical methodology will take the cost in place at the time, multiplied by a markup percentage. • Estimated Total Charges of Procedure/Stays/Visits etc can be obtained by contacting -AJ Karpinski at 218-546-2507. All charges are then subject to Insurance contract payer reductions. To get any accurate representation of patient final cost, I would strongly encourage contacting us to accurately predict final price -costs of services State Mandated Clinic Price Transparency – Average. Charge Medicare Medicaid Cuyuna Regional Medical Center - Clinic Commecial Reim. Office/outpatient visit new Level 1 $190 $76 $45 $34 Office/outpatient visit new Level 2 $396 $130 $75 $57 Office/outpatient visit new Level 3 $574 $186 $107 $82 Office/outpatient visit new Level 4 $708 $283 $163 $126 Office/outpatient visit est Level 1 $96 $36 $22 $16 Office/outpatient visit est Level 2 $187 $76 $44 $34 Office/outpatient visit est Level 3 $424 $127 $73 $56 Office/outpatient visit est Level 4 $549 $187 $108 $83 Office/outpatient visit est Level 5 $624 $245 $145 $112 Per pm reeval est pat infant $300 $176 Non-cov. $77 Prev visit est age 1-4 $347 $184 Non-cov. $82 Prev visit est age 18-39 $432 $205 Non-cov. -

Performance Measures for Upper Gastrointestinal Endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative
Guideline Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative Authors Raf Bisschops1, Miguel Areia2,3, Emmanuel Coron4, Daniela Dobru5, Bernd Kaskas6, Roman Kuvaev7, Oliver Pech8, Krish Ragunath9, Bas Weusten10, Pietro Familiari11, Dirk Domagk12, Roland Valori13, Michal F. Kaminski14, 15, Cristiano Spada11, Michael Bretthauer14, 16, Cathy Bennett17, Carlo Senore18, Mário Dinis-Ribeiro3,19, Matthew D. Rutter20,21 Institutions Institutions are listed at end of article. Bibliography Abbreviations rate, and interval cancers, among others) have DOI http://dx.doi.org/ ! been identified over the last decade [2,3]. Follow- 10.1055/s-0042-113128 CI confidence interval ing the Quality in UGI Endoscopy meeting held in Published online: 22.8.2016 EAC early adenocarcinoma Lisbon in 2013, it was clear that there was a need Endoscopy 2016; 48: 843–864 © Georg Thieme Verlag KG EMR endoscopic mucosal resection to identify performance measures for the UGI Stuttgart · New York ENT ear, nose, and throat tract, and that quality standards could be identi- ISSN 0013-726X ESGE European Society of Gastrointestinal fied although there is a paucity of evidence. This Endoscopy lack of evidence helps however to identify re- Corresponding author Raf Bisschops FAP familial adenomatous polyposis search priorities for the development of clinical Department of GAVE gastric antral vascular ectasia trials that will further validate and substantiate Gastroenterology and HGD high -

Upper Gastrointestinal Endoscopy Policy Number: PG0449 ADVANTAGE | ELITE | HMO Last Review: 11/28/2018
Upper Gastrointestinal Endoscopy Policy Number: PG0449 ADVANTAGE | ELITE | HMO Last Review: 11/28/2018 INDIVIDUAL MARKETPLACE | PROMEDICA MEDICARE PLAN | PPO GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each individual policyholder contract. Paramount applies coding edits to all medical claims through coding logic software to evaluate the accuracy and adherence to accepted national standards. This guideline is solely for explaining correct procedure reporting and does not imply coverage and reimbursement. SCOPE X Professional _ Facility DESCRIPTION Upper gastrointestinal (GI) endoscopy, or esophagogastroduodenoscopy (EGD) is usually performed to evaluate symptoms of persistent upper abdominal pain, nausea, vomiting, and difficulty swallowing or bleeding from the upper GI tract. EGD is more accurate than x-ray films for detecting inflammation, ulcers, or tumors of the esophagus, stomach and duodenum and can detect early cancer, as well as distinguish between benign and malignant conditions when biopsies of suspicious areas are obtained. Esophagogastroduodenoscopy (EGD) uses a flexible fiber-optic scope with a light and camera to examine the upper part of the GI system. The scope is inserted through the mouth into the upper GI tract allowing for direct visualization of the esophagus, stomach, and duodenum through the camera. This document does not address upper gastrointestinal (GI) endoscopy in children, wireless capsule endoscopy, virtual endoscopy or in vivo analysis of gastrointestinal lesions via endoscopy. POLICY Upper gastrointestinal endoscopy does not require prior authorization. Appropriate ICD-10 diagnosis code (as listed below) required for coverage. COVERAGE CRITERIA HMO, PPO, Individual Marketplace, Elite/ProMedica Medicare Plan, Advantage These procedures for adults aged 18 years or older can only be allowed if abnormal signs or symptoms or known disease are present. -

Intraductal Biliopancreatic Imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review
Review 739 Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review Authors Andrea Tringali1, Arnaud Lemmers2, Volker Meves3, Grischa Terheggen4, Jürgen Pohl3, Guido Manfredi5, Michael Häfner6, Guido Costamagna1, Jacques Devière2, Horst Neuhaus4, Fabrice Caillol7, Marc Giovannini7, Cesare Hassan8, Jean-Marc Dumonceau9 Institutions Institutions are listed at end of article. Bibliography This technology review expresses the current biliary strictures/intraluminal lesions, difficult DOI http://dx.doi.org/ view of the European Society of Gastrointestinal biliary stones) and to the setting of tertiary care 10.1055/s-0034-1392584 Endoscopy (ESGE) on the available techniques for centers. Published online: 0.0.2015 intraductal biliopancreatic imaging. Peroral pancreatoscopy may find an indication in Endoscopy 2015; 47: 739–753 © Georg Thieme Verlag KG The three cholangioscopy techniques are de- situations where other imaging modalities (main- Stuttgart · New York scribed: the “dual-operator” and “ single-opera- ly EUS) are inconclusive (i.e. delineation of main ISSN 0013-726X tor” mother-baby approaches using dedicated in- duct intraductal papillary mucinous neoplasia ex- struments, and the “direct” technique using cur- tension, sampling of indeterminate main pancre- Corresponding author rently available ultrathin gastroscopes. atic duct strictures) Andrea Tringali, MD PhD Digestive Endoscopy Unit, The mother-baby method is standardized and re- Intraductal ultrasonography (IDUS) has a poorer -

Endoscopic Management of Primary Sclerosing Cholangitis
842 Barkin JA, et al. , 2017; 16 (6): 842-850 CONCISE REVIEW November-December, Vol. 16 No. 6, 2017: 842-850 The Official Journal of the Mexican Association of Hepatology, the Latin-American Association for Study of the Liver and the Canadian Association for the Study of the Liver Endoscopic Management of Primary Sclerosing Cholangitis Jodie A. Barkin,* Cynthia Levy,** Enrico O. Souto* * University of Miami, Leonard M. Miller School of Medicine, Department of Medicine, Division of Gastroenterology. Miami, Florida, USA. ** University of Miami, Leonard M. Miller School of Medicine, Department of Medicine, Division of Hepatology. Miami, Florida, USA. ABSTRACT Primary sclerosing cholangitis (PSC) remains a rare but potentially devastating chronic, cholestatic liver disease. PSC causes obstruction of intra- and/or extra-hepatic bile ducts by inflammation and fibrosis, leading to biliary obstruction, cirrhosis and portal hypertension with all associated sequelae. The most dreaded consequence of PSC is cholangiocarcinoma, occurring in 10-20% of patients with PSC, and with population-based estimates of a 398-fold increased risk of cholangiocarcinoma in patients with PSC compared to the general population. We use the 4-D approach to endoscopic evaluation and management of PSC based on currently available evidence. After laboratory testing with liver chemistries and high-quality cross-sectional imaging with MRCP, the first D is DDominant stricture diagnosis and evaluation. Second, DDilation of strictures found during ERCP is performed using balloon dilation to as many segments as possible. Third, DDysplasia and cholangiocarcinoma diagnosis is performed by separated brushings for conven- tional cytology and fluorescence in situ hybridization (FISH), and consideration for direct cholangioscopy with SpyGlass™.