Compounded Topical Analgesics for Chronic Pain Abigail E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Baclofen Overdose D
Postgraduate Medical Journal (February 1980) 56, 108-109 Postgrad Med J: first published as 10.1136/pgmj.56.652.108 on 1 February 1980. Downloaded from Baclofen overdose D. J. LIPSCOMB* T. J. MEREDITHt M.B.. M.R.C.P. M.A., M.R.C.P. *Department of Medicine, Peterborough District Hospital, Peterborough PE3 6DA, and tPoisons Unit, Guy's Hospital, London SE] 9RT Summary Case report A 57-year-old woman suffering from multiple sclerosis A 51-year-old woman, who had suffered from took an estimated 1500 mg of baclofen. She became multiple sclerosis for 15 years, was prescribed baclo- deeply unconscious with generalized flaccid muscle fen 40 mg daily as part of her treatment for spastic paralysis and absent tendon reflexes. Toxicological paraplegia. One morning, she was found lying in analysis confirmed the presence of baclofen together bed unconscious and was admitted to hospital. with small amounts of paracetamol and glutethimide. Information given by her husband suggested that an Supportive therapy, including assisted ventilation for overdose of baclofen (consisting of 152 10-mg 3 days, led to complete recovery; anticonvulsant tablets) had been taken about 2 5 hr before being drugs were necessary for the treatment of grand mal found unconscious. On arrival in hospital 90 min fits. The clinical features and treatment of baclofen later, she was deeply unconscious and unresponsive overdose are discussed. to painful stimuli. Respiration was depressed and the cough reflex was absent. She was intubated without Introduction resistance and ventilated; gastric lavage was per-Protected by copyright. Baclofen (Lioresal, Ciba) is widely used in the formed but no tablets were returned in the lavage treatment of muscle spasticity. -

The Use of Intravenous Baclofen As Therapy for the Γ-Hydroxybutyric Acid Withdrawal Syndrome
Research Article Remedy Open Access Published: 12 Jun, 2017 The Use of Intravenous Baclofen as Therapy for the γ-hydroxybutyric Acid Withdrawal Syndrome Marc Sabbe1*, Francis Desmet1 and Sabrina Dewinter2 1Department of Emergency Medicine, University Hospitals, Catholic University of Leuven, Belgium 2Department of Pharmacy, University Hospitals, Catholic University of Leuven, Belgium Abstract Introduction: In this case series with three patients, we introduced baclofen, a γ-aminobutyric acid type B(GABA-B) receptor agonist, for treatment of the γ-hydroxybutyric acid (GHB) withdrawal syndrome. Materials and Methods: Single center case series performed on three patientswith a GHB withdrawal syndrome. They initially received massive doses of benzodiazepines, without sufficient effect. Two patients also received an unsuccessful continuous dexmedetomidine drip. In all patients, intravenous baclofen was started, with an intravenous loading dose between 0.5 and 2 milligrams (mg) to achieve a therapeutic level. Thereafter a continuous intravenous dose between 0.5 and 1 mg per hour for 12 h was administered to maintain a steady state. After that, baclofen was substituted orally with a daily oral dose varying between 20 mg and 40 mg which could be downgraded and stopped over the next days. They all continued to receive a standard benzodiazepine regimen during the baclofen trial. Results and Discussion: Main outcome measurements were the degree of withdrawal symptoms and the need for benzodiazepines during baclofen treatment. In all patients, a significant reduction of the GHB withdrawal syndrome was noted. A standard daily regimen of baseline benzodiazepine dosing between 40 mg and 80 mg diazepam was sufficient. Adverse effects of baclofen use were absent. -

Pricing Sheet
Trusted Service. Proven Savings. Pharmacy: (325) 704-5222 Fax: (325) 777-4819 17 Windmill Circle Suite B Abilene, TX 79606 bigcountry.clarxpharmacy.com SureScript ID: 1171544616 Dermatology Compounds UPDATED 9/3/2020 Medication QTY Price Fluorouracil/ Calcipotriene Cream 4.5/ 0.005% 30 g $55 Fluorouracil Cream 4.5% or 1.5% 30 g $50 Wart Peel Cream (Salicylic Acid/ Fluorouracil 17/ 2%) 30 g $45 Ivermectin Lotion 1.5% 30 g $45 Double Rosacea Cream (Metronidazole/ Ivermectin 1/ 1%) 30 g $55 Triple Rosacea Cream (Azelaic Acid/ Metronidazole/ Ivermectin 15/ 1/ 1%) 30 g $45 Azelaic Acid Cream 17% 30 g $45 Calcipotriene/ Clobetasol Cream 0.005/ 0.045% 60 g $85 Clioquinol/ Hydrocortisone Cream 1/ 1% 30 g $55 Tretinoin Micro Cream 0.033/ 0.055/ 0.09% 45 g $55 Clindamycin/ Tretinoin Micro Cream 1/0.03% 30 g $75 Triamcinolone/ Hydroquinone/ Tretinoin Cream 0.025/ 4/ 0.05% 30 g $55 Hydrocortisone/ Hydroquinone/ Tretinoin Cream 2.5/ 8/ 0.05% 30 g $55 Hydroquinone/ Kojic Acid Cream 12/ 6% or 10/ 3% 30 g $65 Hydroquinone/ Tretinoin Cream 4/ 0.05% 30 g $65 Squaric Acid Solution 0.1 / 0.01 / 0.001% 15 ml $85 Urea Cream 35% 90 g $45 Dapsone 6% Cream 60 g $55 Costmetic Numbing Oint. (Lidocain 23%/Tetracain 7%) 60g $35 General topical compound pricing for other compounds QTY Price Variations to the compounds above will fall into the pricing below. One Active Ingredient 30g $79 Two Active Ingredients 30g $89 Three Active Ingredients 30g $99 *This applies to most compounds (90%) not all, if exceptionally expensive we will call the office to discuss* All cash, no rebates or insurance to mess with! Medicare patients get medications at these great prices too! Free Delivery in 3-5 business days for compounds Page 1 Trusted Service. -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Cream LOTRISONE® Lotion (Clotrimazole and Betamethasone Dipropionate)
LOTRISONE® Cream LOTRISONE® Lotion (clotrimazole and betamethasone dipropionate) FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. NOT RECOMMENDED FOR PATIENTS UNDER THE AGE OF 17 YEARS AND NOT RECOMMENDED FOR DIAPER DERMATITIS. DESCRIPTION LOTRISONE® Cream and Lotion contain combinations of clotrimazole, a synthetic antifungal agent, and betamethasone dipropionate, a synthetic corticosteroid, for dermatologic use. Chemically, clotrimazole is 1–(o-chloro-α,α-diphenylbenzyl) imidazole, with the empirical formula C22H17CIN2, a molecular weight of 344.84, and the following structural formula: Clotrimazole is an odorless, white crystalline powder, insoluble in water and soluble in ethanol. Betamethasone dipropionate has the chemical name 9-fluoro-11β,17,21- trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate, with the empirical formula C28H37FO7, a molecular weight of 504.59, and the following structural formula: 1 LRN# 000370-LOS-MTL-USPI-1 Betamethasone dipropionate is a white to creamy white, odorless crystalline powder, insoluble in water. Each gram of LOTRISONE Cream contains 10 mg clotrimazole and 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone), in a hydrophilic cream consisting of purified water, mineral oil, white petrolatum, cetyl alcohol plus stearyl alcohol, ceteareth-30, propylene glycol, sodium phosphate monobasic monohydrate, and phosphoric acid; benzyl alcohol as preservative. LOTRISONE Cream may contain sodium hydroxide. LOTRISONE Cream is smooth, uniform, and white to off-white in color. Each gram of LOTRISONE Lotion contains 10 mg clotrimazole and 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone), in a hydrophilic base of purified water, mineral oil, white petrolatum, cetyl alcohol plus stearyl alcohol, ceteareth-30, propylene glycol, sodium phosphate monobasic monohydrate, and phosphoric acid; benzyl alcohol as a preservative. -

Topical Therapy As a Treatmentfor Brachioradial
Journal of Case Reports: Open Access Case report Open Access Topical Therapy as a Treatmentfor Brachioradial Pruritis: a Case Report Brianna De Souza M.D, Amy McMichael M.D* Department of Dermatology, Wake Forest University School of Medicine, Winston-Salem, North Carolina *Corresponding author: Amy McMichael, MD Department of Dermatology, Wake Forest Baptist Medical Center,1 Medical Center Blvd, Winston-Salem, NC 27157, Phone: 336-716-7882, Email: [email protected] Received Date: April 12, 2019 Accepted Date: May 06, 2019 Published Date: May 08, 2019 Citation: Brianna De Souza (2019) Topical Therapy as a Treatmentfor Brachioradial Pruritis: a Case Report. Case Reports: Open Access 4: 1-5. Abstract Management of brachioradial pruritus (BRP) presents a formidable challenge to dermatologists and neurologists. BRP is a rare, neurocutaneous condition characterized by sharply localized, chronic pain with associated itching, burning, stinging, and or tingling sensation. Effective care of this patient population is confounded by limitations within the litera- ture, comprised of case series and case reports. We present a case of one middle-aged female with a chronic history of BRP recalcitrant to the following oral therapies: pregabalin, gabapentin, mirtazapine, prednisone, and amitriptyline, as well as topical triamcinolone. After being evaluated in the clinic, the patient was started on combination therapy withKetamine 10%, Amitriptyline 5%, and Lidocaine 5% topical cream to which she responded. Keywords: Brachioradial pruritus, Brachioradial, Pruritus, Neurocutaneous ©2019 The Authors. Published by the JScholar under the terms of the Crea- tive Commons Attribution License http://creativecommons.org/licenses/ by/3.0/, which permits unrestricted use, provided the original author and source are credited. -

Baclofen and Other GABAB Receptor Agents Are Allosteric Modulators of the CXCL12 Chemokine Receptor CXCR4
The Journal of Neuroscience, July 10, 2013 • 33(28):11643–11654 • 11643 Cellular/Molecular Baclofen and Other GABAB Receptor Agents Are Allosteric Modulators of the CXCL12 Chemokine Receptor CXCR4 Alice Guyon,1,2,3 Amanda Kussrow,4 Ian Roys Olmsted,4 Guillaume Sandoz,1,2,5 Darryl J. Bornhop,4* and Jean-Louis Nahon1,2,6* 1Universite´ de Nice Sophia Antipolis, 06103 Nice, France, 2Centre National de la Recherche Scientifique (CNRS), Institut de Pharmacologie Mole´culaire et Cellulaire, UMR 7275, 06560 Valbonne, France, 3Department of Molecular and Cellular Biology and Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, California 94720, 4Vanderbilt Institute of Chemical Biology, Nashville, Tennessee 37235-1822, 5Institute of Biology Valrose, CNRS UMR 7277, INSERM UMR 1091, Laboratories of Excellence, Ion Channel Science and Therapeutics, 06103 Nice, France, 6Station de Primatologie—UPS 846—Centre CNRS, RD56, 13790 Rousset sur Arc, France CXCR4, a receptor for the chemokine CXCL12 (stromal-cell derived factor-1␣), is a G-protein-coupled receptor (GPCR), expressed in the immune and CNS and integrally involved in various neurological disorders. The GABAB receptor is also a GPCR that mediates metabotropic action of the inhibitory neurotransmitter GABA and is located on neurons and immune cells as well. Using diverse approaches, we report novel interaction between GABAB receptor agents and CXCR4 and demonstrate allosteric binding of these agents to CXCR4. First, both GABAB antagonistsandagonistsblockCXCL12-elicitedchemotaxisinhumanbreastcancercells.Second,aGABAB antagonistblocksthepotentiationby CXCL12ofhigh-thresholdCa 2ϩ channelsinratneurons.Third,electrophysiologyinXenopusoocytesandhumanembryonickidneycellline293 ϩ cells in which we coexpressed rat CXCR4 and the G-protein inward rectifier K (GIRK) channel showed that GABAB antagonist and agonist modified CXCL12-evoked activation of GIRK channels. -

Antagonism of Lidocaine Inhibition by Open-Channel Blockers That Generate Resurgent Na Current
4976 • The Journal of Neuroscience, March 13, 2013 • 33(11):4976–4987 Cellular/Molecular Antagonism of Lidocaine Inhibition by Open-Channel Blockers That Generate Resurgent Na Current Jason S. Bant,1,3 Teresa K. Aman,2,3 and Indira M. Raman1,2,3 1Interdepartmental Biological Sciences Program, 2Northwestern University Interdepartmental Neuroscience Program, and 3Department of Neurobiology, Northwestern University, Evanston, Illinois 60208 Na channels that generate resurgent current express an intracellular endogenous open-channel blocking protein, whose rapid binding upon depolarization and unbinding upon repolarization minimizes fast and slow inactivation. Na channels also bind exogenous com- pounds, such as lidocaine, which functionally stabilize inactivation. Like the endogenous blocking protein, these use-dependent inhibi- tors bind most effectively at depolarized potentials, raising the question of how lidocaine-like compounds affect neurons with resurgent Na current. We therefore recorded lidocaine inhibition of voltage-clamped, tetrodotoxin-sensitive Na currents in mouse Purkinje neu- rons, which express a native blocking protein, and in mouse hippocampal CA3 pyramidal neurons with and without a peptide from the   cytoplasmic tail of NaV 4 (the 4 peptide), which mimics endogenous open-channel block. To control channel states during drug exposure, lidocaine was applied with rapid-solution exchange techniques during steps to specific voltages. Inhibition of Na currents by lidocaine was diminished by either the 4 peptide or the native blocking protein. In peptide-free CA3 cells, prolonging channel opening with a site-3 toxin, anemone toxin II, reduced lidocaine inhibition; this effect was largely occluded by open-channel blockers, suggesting that lidocaine binding is favored by inactivation but prevented by open-channel block. -

Salicylic Acid
Treatment Guide to Common Skin Conditions Prepared by Loren Regier, BSP, BA, Sharon Downey -www.RxFiles.ca Revised: Jan 2004 Dermatitis, Atopic Dry Skin Psoriasis Step 1 - General Treatment Measures Step 1 - General Treatment Measures Step 1 • Avoid contact with irritants or trigger factors • Use cool air humidifiers • Non-pharmacologic measures (general health issues) • Avoid wool or nylon clothing. • Lower house temperature (minimize perspiration) • Moisturizers (will not clear skin, but will ↓ itching) • Wash clothing in soap vs detergent; double rinse/vinegar • Limit use of soap to axillae, feet, and groin • Avoid frequent or prolonged bathing; twice weekly • Topical Steroids Step 2 recommended but daily bathing permitted with • Coal Tar • Colloidal oatmeal bath products adequate skin hydration therapy (apply moisturizer • Anthralin • Lanolin-free water miscible bath oil immediately afterwards) • Vitamin D3 • Intensive skin hydration therapy • Limit use of soap to axillae, feet, and groin • Topical Retinoid Therapy • “Soapless” cleansers for sensitive skin • Apply lubricating emollients such as petrolatum to • Sunshine Step 3 damp skin (e.g. after bathing) • Oral antihistamines (1st generation)for sedation & relief of • Salicylic acid itching give at bedtime +/- a daytime regimen as required Step 2 • Bath additives (tar solns, oils, oatmeal, Epsom salts) • Topical hydrocortisone (0.5%) for inflammation • Colloidal oatmeal bath products Step 2 apply od-tid; ointments more effective than creams • Water miscible bath oil • Phototherapy (UVB) may use cream during day & ointment at night • Humectants: urea, lactic acid, phospholipid • Photochemotherapy (Psoralen + UVA) Step 4 Step 3 • Combination Therapies (from Step 1 & 2 treatments) • Prescription topical corticosteroids: use lowest potency • Oral antihistamines for sedation & relief of itching steroid that is effective and wean to twice weekly. -
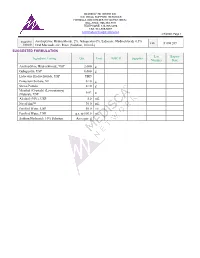
Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 Ml)
MEDISCA® NETWORK INC. TECHNICAL SUPPORT SERVICES FORMULATION CHEMISTRY DEPARTMENT TOLL-FREE: 866-333-7811 TELEPHONE: 514-905-5096 FAX: 514-905-5097 [email protected] 4/7/2020; Page 1 Suggested Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 mL) SUGGESTED FORMULATION Lot Expiry Ingredient Listing Qty. Unit NDC # Supplier Number Date Amitriptyline Hydrochloride, USP 2.000 g Gabapentin, USP 6.000 g Lidocaine Hydrochloride, USP TBD Potassium Sorbate, NF 0.10 g Stevia Powder 0.10 g Menthol (Crystals) (Levorotatory) 0.02 g (Natural), USP Alcohol (95%), USP 5.0 mL NovaFilm™ 30.0 mL Purified Water, USP 50.0 mL Purified Water, USP q.s. to 100.0 mL Sodium Hydroxide 10% Solution As required MEDISCA® NETWORK INC. TECHNICAL SUPPORT SERVICES FORMULATION CHEMISTRY DEPARTMENT TOLL-FREE: 866-333-7811 TELEPHONE: 514-905-5096 FAX: 514-905-5097 [email protected] 4/7/2020; Page 2 Suggested Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 mL) SPECIAL PREPARATORY CONSIDERATIONS Ingredient-Specific Information Light Sensitive (protect from light whenever possible): Amitriptyline Hydrochloride, Gabapentin Hygroscopic (protect from moisture whenever possible): Stevia Powder Narrow Therapeutic Index Lidocaine Hydrochloride Suggested Preparatory Guidelines ■ Non-Sterile Preparation □ Sterile Preparation Processing Error / To account for processing error and pH testing considerations during preparation, it is Testing Considerations: suggested to measure an additional 3 to 5% of the required quantities of ingredients. Special Instruction: This formula may contain one or more Active Pharmaceutical Ingredients (APIs) that may be classified as hazardous, please refer & verify the current NIOSH list of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016. -

(6337) Smartpharmacy.Com
Phone 1.877.811.MEDS (6337) Fax 800.910.7195 SmartPharmacy.com Your prescription has been faxed to SMART PHARMACY Fax: (800) 910-7195 Phone: (877) 811-MEDS (6337) ** SHIPPING IS ALWAYS FREE Your physician has prescribed a topical cream to treat your pain and inflammation. Please review the following information about your prescription. HOW DO I FILL MY PRESCRIPTION? HOW DO I APPLY THE CREAM? • In most cases, your physician’s office will fax your The proper application of the cream is imperative to achieve prescription directly to Smart Pharmacy. A Smart Pharmacy maximum results; therefore, be sure to follow directions on the Registered Pharmacy Technician will begin processing your prescription label. prescription and contact you by phone. • Your prescription will arrive in an easy-to-use pump applicator. • Please leave a good contact number for calling or texting in order to expedite your order. • Clean and dry the affected area prior to applying topical cream with each application. • With your approval, the prescription will be shipped directly to your home or business at no charge. • Use latex gloves or thoroughly wash hands before and after each application. Do not apply to open wounds. Do not touch eyes or WILL INSURANCE COVER MY SMART mouth before washing hands as you may cause burning and irritation. PHARMACY PRESCRIPTION? • Most commercial insurance plans cover topical preparations with a reasonable co-pay. • Apply a liberal amount to affected area and rub in well as directed by your physician, massaging the affected area for 1-2 minutes. • Smart Pharmacy will verify your coverage and discuss your co-pay obligation. -

Drugs That Are Not Covered
Drugs that are Not Covered* Current 10/1/21 In addition to this list, newly marketed prescription medications may not be covered until the Pharmacy & Therapeutics Committee has had an opportunity to review the medication, to determine whether the medication will be covered and if so, which tier will apply based on safety, efficacy and the availability of other products within that class of medications. The current list of newly marketed drugs can be found on our New to Market Drug list. Abilify tablets albuterol HFA inhalers (authorized Apexicon E cream Abilify MyCite tablets generics for ProAir, Proventil, Ventolin Apidra vials Absorica capsules HFA inhalers) Apidra SoloStar injection Absorica LD capsules Aldactone tablets Aplenzin tablets Abstral sublingual tablets Aldara cream Apriso capsules Acanya gel and pump gel Alkindi sprinkle capsules Arava tablets Accupril tablets Allegra Children’s Allergy ODT Arazlo lotion acetaminophen 320.5 mg/caffeine 30 Allegra ODT, suspension and tablets Arestin microspheres mg/dihydrocodeine 16 mg Alltizal tablets Aricept tablets capsulesAciphex tablets alogliptin (authorized generic for Aricept ODT Aciphex Sprinkle capsules Nesina) Arimidex tablets Acticlate tablets alogliptin/metformin tablets (authorized Arixtra injection Active-Prep kits generic for Kazano) ArmonAir Digihaler inhaler Activella tablets alogliptin/pioglitazone (authorized ArmonAir Respiclick inhaler Actonel tablets generic for Oseni) Aromasin tablets Actoplus Met tablets Alphagan P 0.1% eye drops Arthrotec 50 and 75 tablets Actos