The Influence of Residual Sodium on the Catalytic Oxidation of Propane and Toluene Over Co3o4 Catalysts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SAFETY DATA SHEET Nonflammable Gas Mixture: Carbon Dioxide / Carbon Monoxide / Nitric Oxide / Nitrogen / Propane Section 1
SAFETY DATA SHEET Nonflammable Gas Mixture: Carbon Dioxide / Carbon Monoxide / Nitric Oxide / Nitrogen / Propane Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Carbon Dioxide / Carbon Monoxide / Nitric Oxide / Nitrogen / Propane Other means of : Not available. identification Product type : Gas. Product use : Synthetic/Analytical chemistry. SDS # : 002173 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. May increase respiration and heart rate. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Not applicable. Hazards not otherwise : In addition to any other important health or physical hazards, this product may displace classified oxygen and cause rapid suffocation. -

Experimental and Analytical Study of Nitric Oxide Formation During Combustion of Propane in a Jet-Stirred Combustor
NASA Technical Paper 1181 I 1 Experimental and Analytical Study of Nitric Oxide Formation During / Combustion of Propane in a Jet-Stirred Combustor , ' N. T. Wakelyn, Casimir J. Jachimowski, and Charles H. Wilson , TECH LIBRARY KAFB, NM 0134538 NASA Technical Paper 1181 Experimental and Analytical Study of Nitric Oxide Formation During Combustion of Propane in a Jet-Stirred Combustor N. T. Wakelyn, Casimir J. Jachimowski, and Charles H. Wilson Langley Research Center Hampton, Virginia National Aeronautics and Space Administration Scientific and Technical Information Otfice 1978 '8' SUMMARY A jet-stirred combustor, constructed of castable zirconia and with an inconel injector, has been used to study nitric oxide formation in propane-air combustion with residence times in the range from 3.2 to 3.3 msec and equiva lence ratios varying from 0.7 to 1.4. The residence time range was character istic of that found in the primary zones of aircraft turbines. Premixed propane-air formulations were subjected to intense and turbulent backmixed combustion within the cavity formed between the injector and the hemispherical inner walls of the zirconia shell. The volume of the combustor cavity was 12.7 cm3 and the mass loading was maintained in the range from 0.053 to 0.055 g/cm3-sec. Measurements were made of combustor operating tem perature and of nitric oxide concentration. Maximum nitric oxide concentrations of the order of 55 ppm were found in the range of equivalence ratio from 1.0 to 1.1. Nitric oxide concentrations were predicted over the range of equivalence ratio by a computer program which employs a perfectly stirred'reactor (PSR) algorithm with finite-rate kinetics. -

Alternatives to High Propane Prices Interest Expense and the Cost of Utilities Are the Two Largest “Out of Pocket” Expenses Facing Most Broiler Growers
ThePoultry Engineering, Economics & Management NEWSLETTER Critical Information for Improved Bird Performance Through Better House and Ventilation System Design, Operation and Management Auburn University, in cooperation with the U.S. Poultry & Egg Association Issue No 29, May 2004 Alternatives to High Propane Prices Interest expense and the cost of utilities are the two largest “out of pocket” expenses facing most broiler growers. There is little a grower can do to reduce interest costs, short of major mortgage refinancing. However, costs associated with utilities are another matter. The topics we have addressed in most past issues of this newsletter have all dealt with housing and ventilation factors which can result in improved flock performance and in reduced levels of energy being used, which directly reduces costs to the grower. Electricity rates are typically fixed and are highly regulated. However, the price of heating fuel, specifically propane (liquefied petroleum gas or LPG), has varied widely up and down in most recent years, depending on the time of year and numerous supply and demand conditions. Growers can choose What growers should be aware of is that there are ways to reduce the from several good risk that they will have to buy propane at one of those times when the alternatives to paying price is highest. This newsletter explains several alternative methods consistently high prices growers can use to get lower and more stable propane costs over time. for propane. Understanding the Basics of Propane Pricing The most fundamental factor in propane pricing is that propane is basically a fossil fuel, being derived from raw natural gas during the oil refining process. -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product number 1000007900 Product identifier AUTO GLASS QUICK RELEASE AGENT Company information Sprayway, Inc. 1005 S. Westgate Drive Addison, IL 60101 United States Company phone General Assistance 1-630-628-3000 Emergency telephone US 1-866-836-8855 Emergency telephone outside 1-952-852-4646 US Version # 01 Recommended use Not available. Recommended restrictions None known. 2. Hazard(s) identification Physical hazards Flammable aerosols Category 1 Health hazards Serious eye damage/eye irritation Category 2A Specific target organ toxicity, single exposure Category 3 narcotic effects Aspiration hazard Category 1 Environmental hazards Not classified. OSHA defined hazards Not classified. Label elements Signal word Danger Hazard statement Extremely flammable aerosol. May be fatal if swallowed and enters airways. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statement Prevention Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Do not spray on an open flame or other ignition source. Pressurized container: Do not pierce or burn, even after use. Avoid breathing gas. Wash thoroughly after handling. Use only outdoors or in a well-ventilated area. Wear eye/face protection. Response If swallowed: Immediately call a poison center/doctor. If inhaled: Remove person to fresh air and keep comfortable for breathing. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Call a poison center/doctor if you feel unwell. Do NOT induce vomiting. If eye irritation persists: Get medical advice/attention. Storage Store in a well-ventilated place. Keep container tightly closed. Store locked up. -

Fuel Properties Comparison
Alternative Fuels Data Center Fuel Properties Comparison Compressed Liquefied Low Sulfur Gasoline/E10 Biodiesel Propane (LPG) Natural Gas Natural Gas Ethanol/E100 Methanol Hydrogen Electricity Diesel (CNG) (LNG) Chemical C4 to C12 and C8 to C25 Methyl esters of C3H8 (majority) CH4 (majority), CH4 same as CNG CH3CH2OH CH3OH H2 N/A Structure [1] Ethanol ≤ to C12 to C22 fatty acids and C4H10 C2H6 and inert with inert gasses 10% (minority) gases <0.5% (a) Fuel Material Crude Oil Crude Oil Fats and oils from A by-product of Underground Underground Corn, grains, or Natural gas, coal, Natural gas, Natural gas, coal, (feedstocks) sources such as petroleum reserves and reserves and agricultural waste or woody biomass methanol, and nuclear, wind, soybeans, waste refining or renewable renewable (cellulose) electrolysis of hydro, solar, and cooking oil, animal natural gas biogas biogas water small percentages fats, and rapeseed processing of geothermal and biomass Gasoline or 1 gal = 1.00 1 gal = 1.12 B100 1 gal = 0.74 GGE 1 lb. = 0.18 GGE 1 lb. = 0.19 GGE 1 gal = 0.67 GGE 1 gal = 0.50 GGE 1 lb. = 0.45 1 kWh = 0.030 Diesel Gallon GGE GGE 1 gal = 1.05 GGE 1 gal = 0.66 DGE 1 lb. = 0.16 DGE 1 lb. = 0.17 DGE 1 gal = 0.59 DGE 1 gal = 0.45 DGE GGE GGE Equivalent 1 gal = 0.88 1 gal = 1.00 1 gal = 0.93 DGE 1 lb. = 0.40 1 kWh = 0.027 (GGE or DGE) DGE DGE B20 DGE DGE 1 gal = 1.11 GGE 1 kg = 1 GGE 1 gal = 0.99 DGE 1 kg = 0.9 DGE Energy 1 gallon of 1 gallon of 1 gallon of B100 1 gallon of 5.66 lb., or 5.37 lb. -

BENZENE Disclaimer
United States Office of Air Quality EPA-454/R-98-011 Environmental Protection Planning And Standards June 1998 Agency Research Triangle Park, NC 27711 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF BENZENE Disclaimer This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation of use. EPA-454/R-98-011 ii TABLE OF CONTENTS Section Page LIST OF TABLES.....................................................x LIST OF FIGURES.................................................. xvi EXECUTIVE SUMMARY.............................................xx 1.0 PURPOSE OF DOCUMENT .......................................... 1-1 2.0 OVERVIEW OF DOCUMENT CONTENTS.............................. 2-1 3.0 BACKGROUND INFORMATION ...................................... 3-1 3.1 NATURE OF POLLUTANT..................................... 3-1 3.2 OVERVIEW OF PRODUCTION AND USE ......................... 3-4 3.3 OVERVIEW OF EMISSIONS.................................... 3-8 4.0 EMISSIONS FROM BENZENE PRODUCTION ........................... 4-1 4.1 CATALYTIC REFORMING/SEPARATION PROCESS................ 4-7 4.1.1 Process Description for Catalytic Reforming/Separation........... 4-7 4.1.2 Benzene Emissions from Catalytic Reforming/Separation .......... 4-9 4.2 TOLUENE DEALKYLATION AND TOLUENE DISPROPORTIONATION PROCESS ............................ 4-11 4.2.1 Toluene Dealkylation -

Detection and Characterization of Subsurface Dissolved Hydrocarbon Plumes by in Situ Mass Spectrometry – a Demonstration in Th
Detection and characterization of subsurface dissolved hydrocarbon plumes by in situ mass spectrometry – A demonstration in the natural laboratory of the Coal Oil Point seep field. Ira Leifer1,2, Tim Short3, Scott Hiller4, Michael Suschikh1 California Oil Spill Prevention and Response Chevron Technology Workshop San Ramon CA, Feb 26-28 2013 Mission Goals 1. Identify dissolved components under surface oil slicks. 2. Identify and characterize dissolved oil component plumes. 3. Use sidescan sonar to characterize plume source. 4. Test conventional fluorometric detection of dissolved and submerged oil (on a shoestring budget) How a MIMS works in situ Membrane Introduction Mass Spectrometer Dissolved gas transfer across a semipermeable membrane from a sample water flow provides sample into the mass spectrometer for analysis Gases breakup into known fragments, which then are used to identify the incoming gases. Key to calibration is reduction of water vapor from the gas stream entering the mass spectrometer Introduction of Analytes from the Water • MIMS is ideal Column – Passive (except for sample pumping and heating, if desired) – Polydimethylsiloxane (PDMS) or Teflon are most common choices (hydrophobic) – Provides sensitive detection of dissolved gases and volatile organic compounds • Need to mechanically support membrane (hydrostatic pressure) – Porous metal or ceramic frit SRI MIMS Adapted for Underwater In Situ Analysis Microcontroller Embedded PC and other electronics MS electronics (Inficon CPM 200) 200 amu linear quadrupole in vacuum housing w/ heating jacket Turbo pump (Varian/Agilent V81-M) MIMS probe Roughing pump (KNF Neuberger) High pressure membrane interface Portable Underwater Mass Spectrometry • Simultaneous in situ quantification of multiple analytes – Dissolved gases – Light hydrocarbons – Volatile organic compounds • High pressure, direct sample introduction – Under development . -
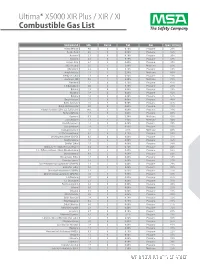
Ultima Infrared Combustible Gas List
Ultima® X5000 XIR Plus / XIR / XI Combustible Gas List Compound LEL Curve Cal Gas Span Setting Acetaldehyde 4.0 8 0.10% Propane 29% Acetic Acid 4.0 3 0.60% Propane 12% Acetone 2.5 8 0.10% Propane 20% Acrolein 2.8 8 0.10% Propane 59% Acrylic Acid 2.4 2 0.60% Propane 10% Allyl Alcohol 2.5 1 2.50% Methane 85% Allylamine 2.2 8 0.10% Propane 18% Amyl Acetate 1.1 1 2.50% Methane 80% t-Amyl Alcohol 1.3 6 0.60% Propane 41% Aromatic 100 0.9 1 2.50% Methane 75% Benzene 1.2 8 0.10% Propane 42% 1,3 Butadiene 2.0 8 0.10% Propane 23% Butane 1.9 4 0.60% Propane 29% Butanol 1.4 6 0.60% Propane 42% Butene 1.6 6 0.60% Propane 57% Butyl Acetate 1.7 6 0.60% Propane 40% Butyl Acrylate 1.5 6 0.60% Propane 45% Butyl Methacrylate 2.0 6 0.60% Propane 33% t-Butyl Peroxide (20% LEL Full Scale) 18.0 3 0.60% Propane 10% Butyraldehyde 1.4 6 0.60% Propane 65% Cumene 0.9 1 2.50% Methane 43% Cyclohexane 1.3 1 2.50% Methane 50% Cyclohexanone 1.1 6 0.60% Propane 74% Cyclopentane 1.5 7 0.60% Propane 31% Cyclopentanone 1.5 1 2.5% Methane 60% 1,2 Dichloroethane 6.2 8 0.10% Propane 14% Dicyclopentadiene (DCPD) 0.8 6 0.60% Propane 55% Diethylamine 1.8 2 0.60% Propane 32% Diethyl Ether 1.9 2 0.60% Propane 38% Difluoro -1- (142b) Chloroethane 6.2 8 0.10% Propane 20% 1,1 - Difluoroethane (152a) Diisobutylene 3.7 2 0.60% Propane 52% Diisobutylene 0.8 2 0.60% Propane 52% Diisopropyl Ether 1.4 6 0.60% Propane 34% Dimethylamine 2.8 2 0.60% Propane 37% Dimethylaminopropylamine (DMAPA) 2.3 2 0.60% Propane 29% Dimethyl Ether 3.4 2 0.60% Propane 32% Dimethyl-ethylamine (DMEA) 2.3 2 0.60% -

Chapter 22 Assignment Answers – Organic Chemistry Unit
Chapter 22 Assignment Answers – Organic Chemistry Unit Chapter 22: 37 – 41, 50, 53, 55, 56, 59, 66, 73 37. pentane: CH3CH2CH2CH2CH3 hexane: CH3CH2CH2CH2CH2CH3 38. (a) propane (b) octane (c) pentane 39. Remember… “alkyl” groups are side groups that branch off a longer-chain hydrocarbon. methyl ̶ CH3 ethyl ̶ CH2CH3 propyl ̶ CH2CH2CH3 40. (a) 2-methylbutane (b) 2,3-dimethylbutane (c) 3-methylhexane 41. Alkane molecules are nonpolar because all of the bonds within the molecule are nonpolar bonds. You can make an alkane molecule polar by adding a functional group to it, however. 50. Both structures are the same, because the alternating double bonds don’t really exist! Remember that the carbon-to-carbon bonds in benzene are all identical. 53. When coal that contains a high percentage of sulfur burns, the major air pollutants SO2 and SO3 are by-products. 55. (a) ethyne (b) propane (c) methylbenzene 56. (a) propyne: (b) cyclohexane: (c) 2-phenylpropane (d) 2,2,4-trimethylpentane 66. Methene does not exist. You only have one carbon in the molecule, so a double bond cannot exist within the molecule. 73. Alkanes: all single bonds between C atoms Alkenes: a double bond exists between C atoms Aromatic: a benzene ring exists within the molecule Cycloalkane: “ring” of single-bonded C atoms Chapter 23 Assignment Answers – Organic Chemistry Unit Chapter 23: # 26, 27, 38, 39, 41, 43, 45, 50, 53 26. “R” indicates a carbon chain or ring. 27. (a) (b) (c) 38. (a) halocarbon: R – X (X is any halogen) (b) ketone: (c) ester: (d) amide: 39. -

Ethane / Nitrous Oxide Mixtures As a Green Propellant to Substitute Hydrazine: Validation of Reaction Mechanism C
Ethane / Nitrous Oxide Mixtures as a Green Propellant to Substitute Hydrazine: Validation of Reaction Mechanism C. Naumann*, C. Janzer, U. Riedel German Aerospace Center (DLR), Institute of Combustion Technology Pfaffenwaldring 38-40, 70569 Stuttgart, Germany Abstract The combustion properties of propellants like ethane / nitrous oxide mixtures that have the potential to substitute hydrazine or hydrazine / dinitrogen tetroxide in chemical propulsion systems are investigated. In support of CFD- simulations of new rocket engines powered by green propellants ignition delay times of ethane / nitrous oxide mixtures diluted with nitrogen have been measured behind reflected shock waves at atmospheric and elevated pressures, at stoichiometric and fuel-rich conditions aimed for the validation of reaction mechanism. In addition, ignition delay time measurements of ethene / nitrous oxide mixtures and ethane / O2 / N2 - mixtures with an O2:N2 ratio of 1:2 as oxidant at the same level of dilution are shown for comparison. Finally, the ignition delay time predictions of a recently published reaction mechanism by Glarborg et al. are compared with the experimental results. Introduction waves at initial pressures of pnominal = 1, 4, and 16 bar. Hydrazine and hydrazine derivatives like The results are compared to ignition delay time monomethyl hydrazine (MMH) and unsymmetrical measurements of stoichiometric C2H4 / N2O mixtures dimethyl hydrazine (UDMH) are used for spacecraft (see [6], [7], [8]). Complementary, ignition delay times propulsion applications in various technological of the corresponding oxygen based reactive mixture contexts despite their drawback of being highly toxic. C2H6 / (1/3 O2 + 2/3 N2) have been measured, too. Today, hydrazine consumption for European space Thereafter, the predictions of a recently published activities is on the order of 2-5 tons per year. -

Enthalpies of Hydrogen-Bonded Butylamine-Chloroform Complexes*
Proc. Indian Acad. Sci. (Chem. Sci.), Vol. 99, Nos 5 & 6, December 1987, pp. 413-417. Printed in India. Enthalpies of hydrogen-bonded butylamine-chloroform complexes* GOPAL PATHAK and SHIRISH PRADHAN* National Chemical Laboratory, Pune 411 008, India MS received 18 June 1987; revised 13 October 1987 Abstract. Enthalpies of mixing of isomeric butylamine with chloroform were determined at 30~ by a Calvet-type microcalorimeter. The system showed highly exothermie behaviour due to hydrogen-bonded complex formation on mixing. The enthaipies of complex formation were calculated by means of a thermo-chemical cycle and the values vary from 1-aminobutane to 2-amino-2-propane complexes. Keywords. calorimetry; amine-chloroform complexes; binary systems; enthalpy of comlD|eX. 1. Introduction The energies of hydrogen-bonded complexes depend on the electron densities of proton-acceptor and -donor atoms of the H-bond forming molecules. Steric and electromeric effects also play important roles in strengthening or weakening the H-bond (Geisler et al 1971). The enthaIpies of H-bonded complexes therefore depend on the functional groups as well as the structure of complex-forming molecules. In our earlier work we have determined the enthalpies of H-bonded complexes between NH2 .... OH, NH .... OH, N .... OH groups and also studied the isomeric effect on the strengths of H-bonds (Pradhan and Mathur 1979; Pradhan 1981; Pradhan and Pathak 1986). In the present communication the work on the enthalpies of complex formation between isomeric butylamines and chloroform has been reported. 2. Experimental The isomeric butylamines (S.D. Chemicals, AR grade) were purified by fractional distillation and the chloroform (S.D. -

Butane Propane
Safety Data Sheet Product: Butane-Propane Mixture Page: 1 of 6 Issued: 12-November-2015 SECTION 1 - IDENTIFICATION: PRODUCT IDENTIFIER AND CHEMICAL IDENTITY The Coleman Company, Inc. 3600 N Hydraulic Wichita, Kansas 67219 Phone: 1 800 835 3278 (bus hours) Chemical Nature: Petroleum Gases, Liquefied Trade Name: Butane-Propane Mixture Product Use: Disposable cartridges for use in portable gas appliances Creation Date: 13-March-2013 Revision Date: 12-November-2015 SECTION 2 - HAZARD IDENTIFICATION UN Number: 2037, Receptacles, small, containing gas (gas cartridges) flammable, without release device, not refillable and not exceeding 1 L capacity Classification: Flammable Gases, Category 1 SUSDP Classification: S6 Hazard DANGER Statements Extremely flammable gas. Harmful if inhaled. Causes skin irritation. Causes serious eye irritation. Precautionary Keep out of the reach of children. Statements Keep container in a well-ventilated place at a temperature not exceeding 48oC. Keep away from heat and ignition sources - No Smoking. In case of fire, use dry chemical, water fog or foam. Use only in well ventilated areas. Wash thoroughly with soap and water after handling and before eating, drinking or using tobacco. In case of insufficient ventilation, wear suitable respiratory equipment. Remove to fresh air immediately. Get medical attention immediately. Do NOT induce vomiting. Get immediate medical attention. If frostbite, call a physician. Hold eyelids apart and flush eyes with plenty of water for at least 15 minutes. Get medical attention. Avoid