(Resucts and Discussion V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Is the Use of Yellow Sticky Trap Detrimental to Natural Enemy Complex of Tea Pests?
American-Eurasian J. Agric. & Environ. Sci., 16 (9): 1597-1601, 2016 ISSN 1818-6769 © IDOSI Publications, 2016 DOI: 10.5829/idosi.aejaes.2016.1597.1601 Is the Use of Yellow Sticky Trap Detrimental to Natural Enemy Complex of Tea Pests? 12Souvik Sen, Sunil Kumar Pathak and 3Maqbool Lyngdoh Suiam 1Tea Research Association, Meghalaya Advisory Centre, Lumnongrim, Dewlieh, Umsning, Pin-793105, Meghalaya, India 2Tea Research Association, Tocklai Tea Research Institute, Jorhat, Pin-785008, Assam, India 3Department of Horticulture, Directorate of Agriculture, Govt. of Meghalaya, Tea Development Centre, Lumnongrim, Dewlieh, Umsning, Pin-793105, Meghalaya, India Abstract: Yellow Sticky Traps are excellent tools for precision monitoring of thrips, jassids, white flies and leaf miners and largely used in the tea plantations of North East India. But the question comes in the tea planters’ mind whether there is any adverse effect of such traps on the natural enemy complex of tea pest. In view of this, the present study was undertaken in organically managed tea garden of Meghalaya having higher diversity and density of natural enemies. The study revealed that there is no negative impact on natural enemy population concerning the use of yellow sticky traps. In addition a clear picture was received on type of arthropods trapped in the sticky traps. Key words: Yellow sticky trap North East India Tea plantation Pest Natural enemy INTRODUCTION submarginata), scale insects and mealy bugs are recognized as minor pests which may also adversely India is the second largest black tea producer in the affect the production of tea bushes [3-5]. The minor status world. North East India contributes more than 60% of the of several pests is due to the action of the natural enemies gross national tea production. -

Budidaya Dan Pasca Panen TEH I Budidaya Dan Pasca Panen TEH
Budidaya dan Pasca Panen TEH i Budidaya dan Pasca Panen TEH Penyusun : Ir. Dedi Soleh Effendi, MS Dr. M. Syakir Dr. M. Yusron Dr. Wiratno Redaksi Pelaksana : - Ir. Jusniarti - Agus Budiharto Desain dan Foto sampul : Agus Budiharto Tata Letak : Agus Budiharto Foto : - Prof. Henkie T. Luntungan - Ir. Dedi Soleh Effendi, MS Pusat Penelitian dan Pengembangan Perkebunan Hak Cipta Dilindungi Undang-undang Budidaya dan Pasca Panen Teh ISBN ii Budidaya dan Pasca Panen TEH Kata Pengantar Dalam rangka mendukung pengembangan teh di Indonesia yang produktivitasnya masih rendah. Puslitbang Perkebunan telah menyusun buku Budidaya dan Pasca Panen Teh dalam upaya meningkatkan produk-tivitas teh rakyat. Isi dari pada buku ini memuat persyaratan tumbuh teh, bahan tanaman, persiapan lahan, penanaman, pengelolaan tanaman, pemang-kasan, pemupukan, hama dan penyakit, pemetikan, pasca panen dan diversifikasi usahatani. Sumber penyusunan buku ini diambil dari hasil-hasil penelitian PPTK yang sudah dipublikasikan, hasil diskusi langsung dengan peneliti PPTK, hasil kunjungan ke Kebun Teh Gambung, Pasir Sarongge dan PTPN VIII Gunung Mas, serta informasi dari media cetak dan internet. Budidaya dan Pasca Panen TEH iii Kepada semua pihak yang terlibat dalam penyusunan buku ini disampaikan terimakasih. Masukan dan saran sangat diharapkan bagi perbaikan buku ini. Semoga buku ini bermanfaat bagi yang membacanya dan bagi upaya pengembangan tanaman teh di Indonesia. Bogor, Nopember 2010. Kepala, Dr. M. Syakir Daftar Isi Kata Pengantar....................................................................................... -

Insect Pests, Part B
9.2.4 Thrips Vietnamese name: Bä c¸nh t¬ Scientific names: Heliothrips haemorrhoidalis, Mycterothrips setiventris, Scirtothrips bispinosus, or Scirtothrips dorsalis (Order Thysanoptera, Family Thripidae). Several of these species may be present at the same time. Other species of thrips sometimes are found on tea. During the recent five years, thrips has become a very big problem for the tea growing locations in Bac Can and Thai Nguyen. Even a few thrips feeding on a bud can lower the quality of the bud, making the dried buds brittle (easy to break) and making the processed tea bitter with a yellow liquor (tea water). Description and Behavior Thrips hide from light, and are concentrated inside folded buds, inside flowers, or on the undersides of the youngest leaves. They are very small, narrow insects shaped like tiny grains of rice (see drawing and picture). Because they are so small, you may see only the damage they cause (see “Plant damage and plant tolerance”, below). The adult thrips is about 0.5 - 1.2 mm long, and is just barely visible with the unaided eye. They range in colour from pale brownish-red to flesh-colour to pale green-yellow. Many do not have wings. When present, the wings are very small and transparent, and are composed of many small light hairs. Nymphs look like small adults with no wings. Eggs are so small that you probably will not be able to see them. Thrips walk very slowly, although they can fly from plant to plant fairly quickly. Life cycle The eggs are inserted singly into the leaf tissue, usually next to a vein. -

Aesa Based Package
AESA BASED PACKAGE TEA Directorate of Plant Protection Quarantine National Institute of Plant Health and Storage Management N. H. IV, Faridabad, Haryana Rajendranagar, Hyderabad, Telaangana DEPARTMENT OF AGRICULTURE AND COOPERATION MINISTRY OF AGRICULTURE GOVERNMENT OF INDIA The AESA based IPM - Tea, was compiled by the NIPHM working group under the Chairmanship of Dr. Satyagopal Korlapati, IAS, DG, NIPHM, and guidance of Shri. Utpal Kumar Singh JS (PP). The package was developed taking into account the advice of experts listed below on various occasions before finalization. NIPHM Working Group: Chairman : Dr. Satyagopal Korlapati, IAS, Director General Vice-Chairmen : Dr. S. N. Sushil, Plant Protection Advisor : Dr. P. Jeyakumar, Director (PHM) Core Members : 1. Er. G. Shankar, Joint Director (PHE), Pesticide Application Techniques Expertise. 2. Dr. O. P. Sharma, Joint Director (A & AM), Agronomy Expertise. 3. Dr. Satish Kumar Sain, Assistant Director (PHM), Pathology Expertise. 4. Dr. Dhana Raj Boina, Assistant Director (PHM), Entomology Expertise. 5. Sri. D. Chatopadhyaya, Assistant Director (PHM), Entomology Expertise. Other Members : 1. Dr. B. S. Sunanda, Assistant Scientific Officer (PHM), Nematology Expertise. Contributions by DPPQ&S Experts: 1. Shri. Ram Asre, Additional Plant Protection Advisor (IPM), 2. Dr. K. S. Kapoor, Deputy Director (Entomology), 3. Dr. Sanjay Arya, Deputy Director (Plant Pathology), 4. Dr. Subhash Kumar, Deputy Director (Weed Science) 5. Dr. C. S. Patni, Plant Protection Officer (Plant Pathology) Contributions by External Experts: 1. Dr. Somanth Roy, Scientist C, Department of Entomology, Tocklai Tea Research Institute, Tea Research Association, Jorhat – 785008, Assam, India 2. Dr. Hitendra Kumar Rai, Senior Scientist, Department of Soil Science & Agricultural Chemistry, College of Agriculture, JNKVV, Jabalpur 3. -

Publication of Detail of the Nepalese Quarantine Pests in Nepal Gazette Completed
List of regulated pests (Quarantine Pests) Publication of detail of the Nepalese quarantine pests in Nepal Gazette Completed. Total of 254 Pests of 18 commodities are now officially declared and regulated as quarantine pests. These pests are: National Plant Quarantine Pests of apple, citrus, potato, ginger,garlic,banana, and coffee of Nepal Quarantine Insect and Mite Pests of Apple in Nepal Prohibition of Additional declaration S.N. Scientific name of regulated insect Order:Family (parts of) the required host Ceratitis capitata (Wiedemann) Phytosanitory Diptera: Tephritidae Fruit 1 (mediterranean fruit fly) Certificate Chrysomphalus aonidum (Linnaeus) Phytosanitory Hemiptera: Diaspididae Fruit 2 (circular scale) Certificate Cydia pomonella Linnaeus Phytosanitory Lepidoptera: Tortricidae Fruit 3 (codling moth) Certificate Dysmicoccus brevipes (Cockerell) Hemiptera: Phytosanitory Fruit 4 (pineapple mealybug) Pseudococcidae Certificate Frankliniella occidentalis(Pergande) Phytosanitory Thysanoptera: Thripidae Fruit 5 (western flower thrips) Certificate Grapholita molesta (Busck) Phytosanitory Lepidoptera: Tortricidae Fruit 6 (oriental fruit moth) Certificate Icerya aegyptiaca Douglas Phytosanitory Hemiptera: Margarodidae Fruit 7 (breadfruit mealybug) Certificate Lepidosaphes ulmi (Linnaeus) Phytosanitory Hemiptera: Margarodidae Fruit 8 (oystershell scale) Certificate Parlatoria pergandii Comstock Phytosanitory Hemiptera: Diaspididae Fruit 9 (chaff scale) Certificate Parthenolecanium corni (Bouche) Phytosanitory Hemiptera: Coccidae Fruit 10 -

8. References
8. REFERENCES Abasa RO. 1972. Field and laboratory studies on the adult of Ascotis selenaria reciprocaria (Wlk.) (Lep.: Geometridae) a pest of arábica coffee in Kenya. Bulletin of Entomological Research. 61:559 563. Abbasi B, Ahmed K, Khalique F, Ayub N, Liu H, Kazmi S, Aftab M. 2007. Rearing the cotton bollworm, Helicoverpa armigera, on a tapioca-based artificial diet. Journal of Insect Science. 7:35. Abdel-Rahman HR, Al-Mozini RN. 2007. Antifeedant and toxic activity of some plant extracts against larvae of cotton leaf worm Spodoptera littoralis (Lepidoptera: Noctuidae). Pakistan Journal of Biological Science. 10:4467 4472. Abdullah M, Sarnthoy O, Chaeychomsri S. 2000. Comparative study of artificial diet and soybean leaves on growth, development and fecundity of beet armyworm, Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae). Kasetsart Journal (Natural Sciences). 34:339 344. Aboshi T, Yoshinaga N, Noge K, Nishida R, Mori N. 2007. Efficient incorporation of unsaturated fatty acids into volicitin-related compounds in Spodoptera litura (Lepidoptera: Noctuidae). Bioscience, Biotechnology and Biochemistry. 71:607 610. Adati T, Nakamura S, Tamo M, Kawazu K. 2004. Effect of temperature on development and survival of the legume pod-borer, Maruca vitrata (Fabricius) (Lepidoptera: Pyralidae) reared on a semi-synthetic diet. Applied Entomology and Zoology. 39(1):139 145. Agnew P, Haussy C, Michalakis Y. 2000. Effect of density and larval competition on selected life history traits of Culex pipens quinquefasciatus (Diptera: Culicidae). Journal of Medical Entomology. 37(5):732 735. Agnew P, Mallorie H, Sidobre C, Michalakis Y. 2002. A minimalist approach to the effects of density-dependent competition on insects life-history traits. -

2. Review of Literature
2. REVIEW OF LITERATURE 2.1 TEA CROP AND ITS PESTS Tea plants have a rhythmic growth pattern, dormancy and flush, coinciding with either management practice or environmental conditions or a combination of both (Manivel 1980). The permanent leaves below the plucking surface are known as maintenance foliage. These maintenance foliage produce photosynthates which are supplied to other parts of a plant, which respire and grow actively with the help of these photosynthates (Kabir 2001). The fresh flush above the maintenance leaves are harvested for tea manufacturing. As mentioned earlier, tea bushes being a monoculture provide habitat for 1031 arthropod species and 82 nematodes species over the world (Chen and Chen 1989). In Asia, tea is affected by a total of 230 species of insects and mites (Muraleedharan 1992). However, Hazarika et al. (1994) reported that in NE India, 173 arthropods and 16 nematodes are considered as pests of tea. Among them few important pest species from plantations of NE India are: a. Sucking insects: Tea green leaf hooper (Emposca flavescens) (Homoptera: Cicadellidae); Tea aphids (Toxoptera auranti) (Homoptera: Aphididae); Tea Mosquito bug (Helopeltis theivora) (Heteroptera: Miridae); Thrips (Taeniothrips setiventris) (Thysanoptera: Thripidae); Scale insects (Lacenium uride) (Hemiptera: Coccidae) b. Borers: Red borer (Zeuzera coffeae) (Lepidoptera: Cossidae); Shot hole borer (Xyleborus fornicates) (Coleoptera: Scolytidae) c. Root feeders: Root grub (Holotricha impressa) (Coleoptera: Scarabaeidae); Termites [Odontotermes obesus, O. parvidens, Nasutitermes sp., Microtermes obesi, 18 Euhamitermes lighti, Synhamitermes quadriceps, Pericapritermes assamensis, Malaysiocapritermes holmgrenii and Heterotermes indicola] (Blatodea: Termitidae) d. Non-insect arthropods (Mites): Red spider mites (Oligonychus coffeae) (Acari: Tetranychidae), Scarlet mite (Brevipalpus californicus) (Acari: Tenuipalpidae), Purple mite (Calacarus carinatus) (Acari: Eriophyidae) e. -

AESA BASED IPM Package TEA
AESA BASED IPM PACKAGE TEA , , Tel : 040-2330 3424 Tel Ministry of Agriculture & Farmers Welfare Ministry of Agriculture & Farmers Welfare Balaji Scan Pvt. Ltd., Important Natural Enemies of Tea Insect & Mite Pests Parasitoids Plants Suitable for Ecological Engineering in Tea Plantation Mymarid wasp Erythmelus helopeltidis Chelonus spp. Alfalfa Sunflower Ocimum spp. Cotesia ruficrus Anagrus flaveolus Tachinid fly Cosmos Spearmint Mustard Predators Marigold C arrot C astor Robber fly Reduviid Pentatomid bug Cowpea Buckwheat Maize Orius spp. Praying mantis G round beetle The AESA based IPM - Tea, was compiled by the NIPHM working group under the Chairmanship of Dr. Satyagopal Korlapati, IAS, DG, NIPHM, and guidance of Shri. Utpal Kumar Singh JS (PP). The package was developed taking into account the advice of experts listed below on various occasions before finalization. NIPHM Working Group: Chairman : Dr. Satyagopal Korlapati, IAS, Director General Vice-Chairmen : Dr. S. N. Sushil, Plant Protection Advisor : Dr. P. Jeyakumar, Director (PHM) Core Members: 1. Er. G. Shankar, Joint Director (PHE), Pesticide Application Techniques Expertise. 2. Dr. O. P. Sharma, Joint Director (A & AM), Agronomy Expertise. 3. Dr. Satish Kumar Sain, Assistant Director (PHM), Pathology Expertise. 4. Dr. Dhana Raj Boina, Assistant Director (PHM), Entomology Expertise. 5. Sri. D. Chatopadhyay, Assistant Director (PHM), Entomology Expertise. Other Member: 1. Dr. B. S. Sunanda, Assistant Scientific Officer (PHM), Nematology Expertise. Contributions by DPPQ&S Experts: 1. Shri. Ram Asre, Additional Plant Protection Advisor (IPM) 2. Shri R. Murali, Deputy Director (Entomology) 3. Dr. Sanjay Arya, Deputy Director (Plant Pathology) 4. Dr. Subhash Kumar, Deputy Director (Weed Science) Contributions by External Experts: 1. -

To Download the Food Plant Database in PDF Format
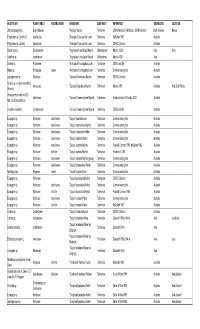
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Abelia spathulata Siebold & Zucc. Caprifoliaceae Acleris askoldana (Christoph) Tortricinae Yasuda 1972 Asia Abelmoschus esculentus (L.) Malvaceae Crocidosema plebejana Zeller Olethreutinae Heinrich 1921; Diakonoff 1982; Nasu & Yasuda 1993 Asia Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Crocidosema plebejana Zeller Olethreutinae Heinrich 1921; Diakonoff 1982 North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Platynota nigrocervina Walsingham Tortricinae MacKay 1962a North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Platynota rostrana (Walker) Tortricinae Heinrich 1921 North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Archips micaceana (Walker) Tortricinae Pholboon 1965; Kuroko & Lewvanich 1993 Asia Moench Abelmoschus esculentus (L.) Malvaceae Archips philippa (Meyrick) Tortricinae BMNH collection Asia India Moench Abelmoschus esculentus (L.) Malvaceae Homona tabescens (Meyrick) Tortricinae Yunus & Ho 1980 Asia Malaysia Moench Abelmoschus esculentus Moench Malvaceae Crocidosema plebejana Zeller Olethreutinae Fletcher 1932 Asia India (as Hibiscus) Abelmoschus esculentus Moench Thaumatotibia leucotreta (Meyrick) (as Malvaceae Olethreutinae Whittle 1984 Africa (as Hibiscus) Cryptophlebia) Abies alba Mill. Pinaceae Acleris variana (Fernald) Tortricinae Meyrick MS 1938 North America Abies alba Mill. Pinaceae Archips oporana (Linnaeus) Tortricinae Bradley et al. 1973 Europe Abies -

Descriptors for Tea (<I>Camellia Sinensis</I>)
Descriptors for Tea (Camellia sinensis) netic t Ge Res lan ou P rc al e n s o I ti n a s t n i r t u e t t e n I IPGRI List of descriptors Pear * (E) 1983 Pearl millet (E,F) 1993 Almond (revised) * (E) 1985 Phaseolus acutifolius (E) 1985 Apple (E) 1982 Phaseolus coccineus * (E) 1983 Apricot * (E) 1984 Phaseolus vulgaris * (E) 1982 Avocado (E,S) 1995 Pigeonpea (E) 1993 Bambara groundnut (E) 1987 Pineapple (E) 1991 Banana (revised) * (E,F,S) # 1996 Pistachio (E,F)# 1997 Barley (E) 1994 Plum * (E) 1985 Beta (E) 1991 Potato variety * (E) 1985 Black pepper (E,S) 1995 Quinoa * (E) 1981 Brassica and Raphanus (E) 1990 Rice * (E) 1980 Brassica campestris L. (E) 1987 Rye and Triticale * (E) 1985 Buckwheat (E) 1994 Safflower * (E) 1983 Capsicum (E,S) 1995 Sesame * (E) 1981 Cardamom (E) 1994 Setaria italica and S. pumilia (E) 1985 Cashew (E) 1986 Sorghum (E,F) 1993 Cherry * (E) 1985 Soyabean * (E,C) 1984 Chickpea (E) 1993 Strawberry (E) 1986 Citrus (E) 1988 Sunflower * (E) 1985 Coconut (E) 1992 Sweet potato (E,F,S) 1991 Coffee (E,F,S) # 1996 Tomato (E,F,S) # 1996 Colocasia * (E) 1980 Tropical fruit * (E) 1980 Cotton (Revised) (E) 1985 Vigna aconitifolia and V. trilobata (E) 1985 Cowpea (E) 1983 Vigna mungo and V. radiata (Revised) * (E)1985 Cultivated potato * (E) 1977 Walnut (E) 1994 Echinochloa millet * (E) 1983 Wheat (Revised) * (E) 1985 Eggplant (E,F) 1990 Wheat and Aegilops * (E) 1978 Faba bean * (E) 1985 White Clover (E) 1992 Finger millet (E) 1985 Winged bean * (E) 1979 Forage grass * (E) 1985 Xanthosoma (E) 1989 Forage legumes * (E) 1984 Yams * (E) 1980 Grape * (E) 1983 Groundnut (E,F,S) 1992 Kodo millet * (E) 1983 IPGRI publications are available free of Lentil * (E) 1985 charge to the libraries of genebanks, Lima bean * (E) 1982 university departments, research institutions, Lupin/Lupinos * (E,S) 1981 etc. -

Taxonomy, Bionomics and Predatory Potential of Eocanthecona Concinna (Walker) (Hemiptera: Pentatomidae: Asopinae)
Journal of Biological Control, 32(2): 81-86, 2018, DOI: 10.18311/jbc/2018/18213 Volume: 32 No. 2 (June 2018) Research Article Taxonomy, bionomics and predatory potential of Eocanthecona concinna (Walker) (Hemiptera: Pentatomidae: Asopinae) K. K. SRIKUMAR1, S. SALINI2*, S. SMITHA1, B. SURESH KUMAR1 and B. RADHAKRISHNAN1 1UPASI Tea Research Foundation, Valparai, Coimbatore - 642127, Tamil Nadu, India 2National Bureau of Agricultural Insect Resources, Bangalore - 560024, Karnataka, India *Corresponding authors E-mail: [email protected] ABSTRACT: Eocantheocna concinna (Walker) is recorded as an important predator of lepidopteran pests in tea plantations of South India. It is described for the first time based on male and female genitalia. Biology of this predator was studied on Corcyra cephalonica larvae. Egg incubation period was 14.3 ± 0.4 days. Five nymphal instars were developed in a period of 33–36 days. Feeding efficacy of different instars of E. concinna was evaluated on third instar tea looper, Biston suppressaria. Results showed fourth and fifth instars of E. concinna attacked faster on B. suppressaria. The study describes E. concinna as a potential predator of various lepidopteran pests in tea plantations and a promising candidate for biological control of looper pests. KEY WORDS: Biology, predatory stink bug, predatory efficiency, lepidopteran pests, taxonomy (Article chronicle: Received: 11-11-2017; Revised: 15-04-2018; Accepted: 15-05-2018) INTRODUCTION of Maharashtra (Waghmare and Gaikwad, 2017). There is no report on the biology and predatory potential of this ef- The members of the subfamily Asopinae, are com- ficient predator. monly known as predatory stink bugs, characterized by a strong four segmented rostrum. -
Sorted by Moth Species
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Jatropha gossypifolia Euphorbiaceae "Amorbia" depicta Tortricinae CSIRO Mexican Field Station; USNM collection North America Meixco Polystichum sp. ("prolifera") Aspidiaceae "Anisogona" placoxantha Lower Tortricinae McQuillan 1992 Australia Polystichum sp. (as fern) Aspidiaceae "Anisogona" placoxantha Lower Tortricinae CSIRO Collection Australia Cordia myxa L. Boraginaceae "Argyroploce" cenchropis Meyrick Olethreutinae Meyrick 1920a Asia India Acanthus sp. Acanthaceae "Argyroploce" vinculigera Meyrick Olethreutinae Meyrick 1939 Asia Banksia sp. Proteaceae "Arotrophora" cosmoplaca Lower Tortricinae CSIRO card file Australia Hakea sp. Proteaceae leaves "Arotrophora" cosmoplaca Lower Tortricinae Common rearing files Australia Leptospermum sp. Myrtaceae "Cacoecia" desmotana Meyrick Tortricinae CSIRO Collection Australia Senecio sp. (as plant resembling Asteraceae "Cacoecia" jugicolana Meyrick Tortricinae Meyrick 1881 Australia New South Wales Senecio) Osteospermum ecklonis (DC.) Asteraceae "Cacoecia" mnemosynana Meyrick Tortricinae Herbison-Evans & Crossley 2003 Australia Norl. (as Dimorphotheca) Goodenia ovata Sm. Goodeniaceae "Cacoecia" mnemosynana Meyrick Tortricinae CSIRO card file Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" ceramica Lower Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" cnaphalodes Meyrick Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" constrictana