Effects on Individuals and Populations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE ECONOMIC EFFECTS of a DECLINING POPULATION by FRANCOIS LAFITTE Itstroduction I5-64) Will Grow Older
THE ECONOMIC EFFECTS OF A DECLINING POPULATION By FRANCOIS LAFITTE Itstroduction I5-64) will grow older. Between I89I and AWELL-CONCEIVED population I937 its average age rose by 24 years, from policy cannot be elaborated unless 34-3 to 36-9 years. In the coming forty the most careful and objective assess- years Glass's projections suggest a further ment of the consequences of present popula- ageing of between 3j and 6 years. Wil this. tion trends is first attempted. If we wish to affect the productivity of the worker ad- modify the British demographic situation versely ? We do not know, but, since the' we have to know why it needs modifying, whole trend of industrial technique is away and in what direction it should be guided. from types of work involving great physical The consequences of differential fertility strength, I doubt whether any possible and mortality are the main concern of deterioration of productivity due to ageing eugenists, and less attention has been of the working population could not be made devoted to the possible effects of changes in up for by improvements in health and work- age composition and total numbers. The ing conditions. In any case, productivity present article is limited to a discussion of per operative employed in Britain rose by the latter in relation to Britain's economic 7 per cent in I924-30 and by at least 20 per future, and is written in lieu of a review of cent in I930-35, whilst in the U.S.A. physical W. B. Reddaway's The Economics of a output per man-hour in industry rose by 27 Declining Population (I939)*, the first sys- per cent in I929-35. -

Who Fears and Who Welcomes Population Decline?
Demographic Research a free, expedited, online journal of peer-reviewed research and commentary in the population sciences published by the Max Planck Institute for Demographic Research Konrad-Zuse Str. 1, D-18057 Rostock · GERMANY www.demographic-research.org DEMOGRAPHIC RESEARCH VOLUME 25, ARTICLE 13, PAGES 437-464 PUBLISHED 12 AUGUST 2011 http://www.demographic-research.org/Volumes/Vol25/13/ DOI: 10.4054/DemRes.2011.25.13 Research Article Who fears and who welcomes population decline? Hendrik P. van Dalen Kène Henkens © 2011 Hendrik P. van Dalen & Kène Henkens. This open-access work is published under the terms of the Creative Commons Attribution NonCommercial License 2.0 Germany, which permits use, reproduction & distribution in any medium for non-commercial purposes, provided the original author(s) and source are given credit. See http:// creativecommons.org/licenses/by-nc/2.0/de/ Table of Contents 1 Introduction 438 2 Population decline: Stylized facts and forecasts 440 3 Population decline and theory 444 3.1 Negative consequences of decline 444 3.2 Positive consequences of decline 447 3.3 The tension between immigration and population decline 448 4 Data and method 449 5 Explaining population size preferences 451 6 Conclusion and discussion 457 7 Acknowledgements 459 References 460 Appendix: Properties of scale variables 463 Demographic Research: Volume 25, Article 13 Research Article Who fears and who welcomes population decline? Hendrik P. van Dalen1 Kène Henkens2 Abstract European countries are experiencing population decline, and the tacit assumption in most analyses is that this decline may have detrimental effects on welfare. In this paper, we use a survey conducted in the Netherlands to find out whether population decline is always met with fear. -

New Perspectives on Tibetan Fertility and Population Decline Author(S): Melvyn C
New Perspectives on Tibetan Fertility and Population Decline Author(s): Melvyn C. Goldstein Source: American Ethnologist, Vol. 8, No. 4 (Nov., 1981), pp. 721-738 Published by: Blackwell Publishing on behalf of the American Anthropological Association Stable URL: http://www.jstor.org/stable/643961 Accessed: 11/10/2010 09:36 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=black. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Blackwell Publishing and American Anthropological Association are collaborating with JSTOR to digitize, preserve and extend access to American Ethnologist. http://www.jstor.org new perspectives on Tibetan fertility and population decline MELVYN C. -

Causes of Urban Shrinkage: an Overview of European Cities Stephen Platt
Causes of Urban Shrinkage: an overview of European cities Stephen Platt Reference: Platt S (2004) Causes of Urban Shrinkage: an overview of European cities. COST CIRES Conference, University of Amsterdam 16-18 February COST CIRES Conference, University of Amsterdam 16-18 February Causes of Urban Shrinkage: an overview of European cities Stephen Platt A number of interrelated factors contribute to or trigger urban shrinkage in European cities. In general there are three principal widespread structural causes of urban decline – economic, social and demographic change. Climate change may also come to play an increasingly role in migration, but to date environmental factors are not a significant cause of shrinkage. Secondary outcomes, for example the migration of young or highly skilled individuals, poorer service provision, regional specialisation or house price differentials, may exacerbate or contribute to further shrinkage. What might be considered a leading factor, however, and what is merely a consequence will depend on the particular case. There is also a scalar dimension at work. Economic restructuring is a global phenomenon and occurs all over the western world. Lower fertility is wide spread all over Europe, but suburbanisation is regional phenomenon. Interestingly the degree of shrinkage varies between cities in the same region and so cannot solely be explained by such macro factors as de- industrialisation and lower fertility. Local characteristics, for example policy initiatives, blurred property rights in the centre of some Eastern European cities, may also contribute to these different levels of shrinkage. Finally, a distinction should be made between the causes of shrinkage for different types and sizes of settlements. -

The Malthusian Economy
The Malthusian Economy Economics 210a January 18, 2012 • Clark’s point of departure is the observation that the average person was no better off in 1800 than in 100,000 BC. – As Clark puts it on p.1. of his book, “Life expectancy was no higher in 1800 than for hunter-gatherers.” – Something changed after that of course. But this is for later in the course….. 2 • Clark’s point of departure is the observation that the average person was no better off in 1800 than in 100,000 BC. – How could he possibly know this? 3 Various forms of evidence, but first and foremost that on heights • There is little sign in modern populations of any genetically determined differences in potential stature, except for some rare groups such as the pygmies of Central Africa. • But nutrition does influence height. • In addition to the direct impact of nutrition on human development, episodes of ill health during growth phases can stop growth, and the body catches up only partially later on. And nutrition is an important determinant of childhood health. • As Clark puts it, “stature, a measure of both the quality of diet and of children’s exposure to disease, was [as high or] higher in the Stone Age than in 1800.” – This is a pretty striking observation. How are we to understand it? 4 The standard framework for doing so is the Malthusian model • Thomas Robert Malthus was born into a wealthy family in 1766, educated at Cambridge, and became a professor at Cambridge and eventually an Anglican parson. • His students referred to him as Pop Malthus (“Pop” for population). -

Reflection Very Long Range Global Population Scenarios to 2300 And
DEMOGRAPHIC RESEARCH VOLUME 28, ARTICLE 39, PAGES 1145-1166 PUBLISHED 30 MAY 2013 http://www.demographic-research.org/Volumes/Vol28/39/ DOI: 10.4054/DemRes.2013.28.39 Reflection Very long range global population scenarios to 2300 and the implications of sustained low fertility Stuart Basten Wolfgang Lutz Sergei Scherbov © 2013 Stuart Basten, Wolfgang Lutz & Sergei Scherbov. This open-access work is published under the terms of the Creative Commons Attribution NonCommercial License 2.0 Germany, which permits use, reproduction & distribution in any medium for non-commercial purposes, provided the original author(s) and source are given credit. See http:// creativecommons.org/licenses/by-nc/2.0/de/ Table of Contents 1 Introduction 1146 2 Could global fertility levels fall to well below population 1147 replacement level? 3 Method 1151 4 Results 1152 5 Conclusions 1154 6 Acknowledgement 1156 References 1157 Appendix 1: Detailed results of alternative global population 1162 projections to 2300 Appendix 2: Input data, code and method of calculation 1165 Demographic Research: Volume 28, Article 39 Reflection Very long range global population scenarios to 2300 and the implications of sustained low fertility Stuart Basten1 Wolfgang Lutz2 Sergei Scherbov3 Abstract BACKGROUND Depending on whether the global level of fertility is assumed to converge to the current European TFR (~1.5) or that of Southeast Asia or Central America (~2.5), global population will either decline to 2.3-2.9 billion by 2200 or increase to 33-37 billion, if mortality continues to decline. Furthermore, sizeable human populations exist where the voluntarily chosen ideal family size is heavily concentrated around one child per woman with TFRs as low as 0.6-0.8. -
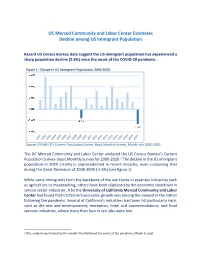
Immigrant Population Decline Has Not Only Been Higher in the Past Twenty Years Than Any Other Year—It Is Even Higher in California (-6.2%) (See Table 1)
UC Merced Community and Labor Center Estimates Decline among US Immigrant Population Recent US Census Bureau data suggest the US immigrant population has experienced a sharp population decline (2.6%) since the onset of the COVID-19 pandemic. Figure 1. Change in US Immigrant Population, 2000-2020 Source: IPUMS-CPS Current Population Survey, Basic Monthly Survey, March-July 2000-2020 The UC Merced Community and Labor Center analyzed the US Census Bureau’s Current Population Survey- Basic Monthly Survey for 2000-2020.1 The decline in the US immigrant population in 2020 (-2.6%) is unprecedented in recent decades, even surpassing that during the Great Recession of 2008-2009 (-1.6%) (see figure 1). While some immigrants form the backbone of the workforce in essential industries such as agriculture or meatpacking, others have been displaced by the economic slowdown in service sector industries. A by the University of California Merced Community and Labor Center had found that California’s economic growth was among the slowest in the nation following the pandemic. Several of California’s industries had been hit particularly hard, such as the arts and entertainment, recreation, hotel and accommodations, and food services industries, where more than four in ten jobs were lost. 1 (The analysis was limited to the months that followed the onset of the pandemic, March to July). Table 1. Immigrant Population, US and California 2019-2020 2019 2020 Change Change % California 10,344,805 9,702,784 -642,021 -6.2% Non-California US 34,965,435 34,434,190 -531,246 -1.5% Total US 45,310,240 44,136,974 -1,173,266 -2.6% Source: IPUMS-CPS Current Population Survey, Basic Monthly Survey, March-July, 2000- 2020 The new analysis finds that immigrant population decline has not only been higher in the past twenty years than any other year—it is even higher in California (-6.2%) (see table 1). -

Chapter 11 Population Dynamics
APES Chapter 4 Human Population Factors in human population size A. Population Growth =(Births + immigration) - (deaths + emigration) B. When factors are stable ZPG = Zero population Growth C. Use Crude birth and death rate - # per 1000 people D. Rate of change % = birth rate – death rate x 100 1,000 people E. World has slowed population rate but still growing very fast F. Fertility rates 1. Replacement level fertility – couple has 2.1 children 2. Total Fertility rate – TFR – estimate of number of children a woman would have under current age specific birth rates a. better measure b. different in different parts of the world - developed 1.6 - developing 3.4 in developing G. Fertility rates in US – more of a problem because of Americans high resource use. 1. drop in TFR but population still growing – Why? - Large number of baby boomers still in childbearing years - Increase in number of teen mothers US has highest rate of any industrialized countries UN studies say US teens not more sexually active just less likely to know how to prevent pregnancies or less willing to use them. 77% of all teen mothers go on welfare within 5 years - Higher fertility rates non Caucasian mothers - High levels of legal and illegal immigrants – accounts for more than 40% of growth H. Factors that affect Birth and fertility rates 1. level of education and affluence 2. Importance of children in the work force 3. Urbanization 4. Cost of raising and educating children 5. Educational and Employment opportunities for women 6. Infant mortality rates 7. Average age of marriage 8. -

Ethical Implications of Population Growth and Reduction Tiana Sepahpour [email protected]
Fordham University Masthead Logo DigitalResearch@Fordham Student Theses 2015-Present Environmental Studies Spring 5-10-2019 Ethical Implications of Population Growth and Reduction Tiana Sepahpour [email protected] Follow this and additional works at: https://fordham.bepress.com/environ_2015 Part of the Ethics and Political Philosophy Commons, and the Natural Resources and Conservation Commons Recommended Citation Sepahpour, Tiana, "Ethical Implications of Population Growth and Reduction" (2019). Student Theses 2015-Present. 75. https://fordham.bepress.com/environ_2015/75 This is brought to you for free and open access by the Environmental Studies at DigitalResearch@Fordham. It has been accepted for inclusion in Student Theses 2015-Present by an authorized administrator of DigitalResearch@Fordham. For more information, please contact [email protected]. Ethical Implications of Population Growth and Reduction Tiana Sepahpour Fordham University Department of Environmental Studies and Philosophy May 2019 Abstract This thesis addresses the ethical dimensions of the overuse of the Earth’s ecosystem services and how human population growth exacerbates it, necessitating an ethically motivated reduction in human population size by means of changes in population policy. This policy change serves the goal of reducing the overall global population as the most effective means to alleviate global issues of climate change and resource abuse. Chapter 1 draws on the United Nations’ Millennium Ecosystem Assessment and other sources to document the human overuse and degradation of ecosystem services, including energy resources. Chapter 2 explores the history of energy consumption and climate change. Chapter 3 examines the economic impact of reducing populations and how healthcare and retirement plans would be impacted by a decrease in a working population. -

Analysis of Inter-Relationships Between Urban Decline and Urban Sprawl in City-Regions of South Korea
sustainability Article Analysis of Inter-Relationships between Urban Decline and Urban Sprawl in City-Regions of South Korea Uijeong Hwang 1 and Myungje Woo 2,* 1 School of City and Regional Planning, Georgia Institute of Technology, 245 4th St NW, Atlanta, GA 30332, USA; [email protected] 2 Department of Urban Planning and Design, University of Seoul, 163, Seoulsiripdae-ro, Dongdaemun-gu, Seoul 02504, Korea * Correspondence: [email protected]; Tel.: +82-2-6490-2803 Received: 1 January 2020; Accepted: 18 February 2020; Published: 22 February 2020 Abstract: This paper identifies inter-relationships between the urban decline in core areas and urban sprawl in hinterlands using 50 city-regions of South Korea. We measured decline- and sprawl-related indicators and estimated a simultaneous equations model using Three-Stage Least Squares. The results show that population decline and employment decline have a different relationship with urban sprawl. While population decline has a negative impact on the urban sprawl in the density aspect, employment decline worsens the urban sprawl in the morphological aspect. Another result suggests that the difference is related to declining patterns of population and employment. Cities that are experiencing population decline in the core area are likely to lose population in their hinterlands as well. On the other hand, the employment decline in the core area shows a positive correlation with employment growth in hinterlands. The results imply that suburbanization of jobs and the inefficient land use exacerbate the urban sprawl in the morphological aspect. Thus, local governments should pay attention to migration patterns of employment and make multi-jurisdictional efforts. -

Executive Summary
EXECUTIVE SUMMARY The United Nations Population Division monitors fertility, mortality and migration trends for all countries of the world, as a basis for producing the official United Nations population estimates and projections. Among the demographic trends revealed by those figures, two are particularly salient: population decline and population ageing. Focusing on these two striking and critical trends, the present study addresses the question of whether replacement migration is a solution to declining and ageing populations. Replacement migration refers to the international migration that would be needed to offset declines in the size of population and declines in the population of working age, as well as to offset the overall ageing of a population. The study computes the size of replacement migration and investigates the possible effects of replacement migration on the population size and age structure for a range of countries that have in common a fertility pattern below the replacement level. Eight countries are examined: France, Germany, Italy, Japan, Republic of Korea, Russian Federation, the United Kingdom of Great Britain and Northern Ireland and the United States of America. Two regions are also included: Europe and the European Union. The time period covered is roughly half a century, from 1995 to 2050. According to the United Nations population projections (medium variant), Japan and virtually all the countries of Europe are expected to decrease in population size over the next 50 years. For example, the population of Italy, currently 57 million, is projected to decline to 41 million by 2050. The population of the Russian Federation is expected to decrease from 147 million to 121 million between 2000 and 2050. -

The Impact of Population Momentum on Future Population Growth
October 2017 No. 2017/4 The impact of population momentum on future population growth 1. What is population momentum? of the World Population Prospects: the instant-replacement- fertility variant, the constant-mortality variant and the zero- For any population, changes over time in its size and migration variant. composition are driven by levels and trends of fertility, mortality and migration. Additionally, a fourth element, the 3. Contribution of population momentum to the age structure of the population, also has an important future growth of the world’s population impact on population trends, including for trajectories of Under the assumptions of the momentum variant, the growth or decline. world’s population would continue to increase in the Thanks to a phenomenon known as population coming years and decades, reaching 8.3 billion in 2030 and momentum, a youthful population with constant levels of 8.9 billion in 2050. Thereafter, the global population would mortality and a net migration1 of zero continues to grow stabilize at around 9 billion. Compared to an estimate of even when fertility remains constant at the replacement around 7.4 billion for 2015, an additional 1.5 billion persons level.2 In this situation, a relatively youthful age structure would thus be added to the world’s population by 2050, promotes a more rapid growth, because the births being even if fertility were to reach the replacement level instantly produced by the relatively large number of women of and if mortality were to remain constant at levels observed reproductive age outnumber the deaths occurring in the in 2010-2015.