Imported Skin Diseases Imported Skin Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vectorborne Zoonoses: Break-Out Session Epidemiology and Laboratory Capacity Workshop – Oct
Texas Department of State Health Services Vectorborne Zoonoses: Break-out Session Epidemiology and Laboratory Capacity Workshop – Oct. 2018 DSHS Zoonosis Control Branch Session Topics Texas Department of State Health Services • NEDSS case investigation tips • Lyme disease • Rickettsial diseases • Arboviral diseases ELC 2018 - Vectorborne Diseases 2 Texas Department of State Health Services Don’t be a Reject! Helpful tips to keep your notification from being rejected ELC breakout session October 3, 2018 Kamesha Owens, MPH Zoonosis Control Branch Texas Department of State Health Services Objectives • Rejection Criteria • How to document in NBS (NEDSS) • How to Report Texas Department of State Health Services 10/3/2018 ELC 2018 - Vectorborne Diseases 4 Rejection Criteria Texas Department of State Health Services Missing/incorrect information: • Incorrect case status or condition selected • Full Name • Date of Birth • Address • County • Missing laboratory data 10/3/2018 ELC 2018 - Vectorborne Diseases 5 Rejection Criteria continued Texas Department of State Health Services • Inconsistent information • e.g. Report date is a week before onset date • Case investigation form not received by ZCB within 14 days of notification • ZCB recommends that notification not be created until the case is closed and the investigation form has been submitted 10/3/2018 ELC 2018 - Vectorborne Diseases 6 Rejection Criteria continued Texas Department of State Health Services • Condition-specific information necessary to report the case is missing: • Travel history for Zika and other non-endemic conditions • Evidence of neurological disease for WNND case • Supporting documentation for Lyme disease case determination 10/3/2018 ELC 2018 - Vectorborne Diseases 7 How to Document in NBS (NEDSS) Do Don’t Add detailed comments in designated Leave us guessing! comments box under case info tab. -

Carabid Beetles Collected from Vegetable Ecosystem
Journal of Pharmacognosy and Phytochemistry 2018; 7(6): 1581-1590 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2018; 7(6): 1581-1590 Carabid beetles collected from vegetable Received: 16-09-2018 Accepted: 18-10-2018 ecosystem Phunu Mili Department of Entomology, Phunu Mili, Anjumoni Devee and Dilip Kumar Saikia Assam Agricultural University, Jorhat, Assam, India Abstract The work on 'Carabid complex of horticultural orchards' was conducted in the Experimental Farm, Anjumoni Devee Department of Horticulture, Assam Agricultural University, Jorhat-13, during the year 2014-2015 and Department of Entomology, Assam Agricultural University, 2015-16 to give a comprehensive information of carabids found in horticultural crops. Carabids were Jorhat, Assam, India collected by pitfall trap, light trap, sweep net and hand picking from okra, brinjal, cabbage, cucumber and bean. Total 12 species of carabids belonging to 7 genera viz., Clivina, Scarites, Harpalus, Pherosophus, Dilip Kumar Saikia Pterostichus, Chlaenius, and Sparostes under 6 tribes- Clivinini, Scaritini, Harpalini, Brachinini, Department of Entomology, Pterostichini and Chlaeniini and 5 subfamily (Scaritinae, Harpalinae, Brachininae, Pterostichinae and Assam Agricultural University, Licininae) were identified by following published Keys and literature and described on the basis of Jorhat, Assam, India observed morphological characters. Among these species, 3 under Clivina viz., C. assamensis, C. memnonia, C. lobata and 2 under Scarites, Harpalus and Pherosophus each viz., S. indus, S. inconspicuous, H. rufipes, H. calceatus, P. occipitalis and Pherosophus sp. From Pterostichus, Chlaenius and Sparostes, there was one species of each genus viz., Pterostichus madidus, C. bimaculatus and Sparostes striatulus. Highest collection of carabids were obtained from pitfall trap (46%) followed by light trap (42%). -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -
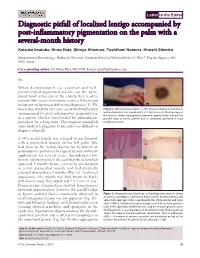
Diagnostic Pitfall of Localized Lentigo Accompanied by Post-Inflammatory
Letter to the Editor Diagnostic pitfall of localized lentigo accompanied by post-inflammatory pigmentation on the palm with a several-month history Keisuke Imafuku, Hiroo Hata, Shinya Kitamura, Toshifumi Nomura, Hiroshi Shimizu Department of Dermatology, Hokkaido University Graduate School of MedicineNorth 15, West 7, Kita-ku, Sapporo 060- 8638, Japan Corresponding author: Dr. Hiroo Hata, MD, PhD, E-mail: [email protected] Sir, When dermatologists see acquired and well- circumscribed pigmented macules on the palm, dorsal hand or forearm of the elderly, they tend to consider blue nevus, hematoma, nevus cell nevus and malignant melanoma as differential diagnoses [1]. We a b herein described the very rare case of localized lentigo Figure 1: Clinical manifestations. a. The macule is brown to dark black, accompanied by post-inflammatory pigmentation well-demarcated, flat, round and 3 x 4 mm in size. b. Dermoscopy of the macule shows homogenous brownish pigmentation without the in a patient who has been treated for palmoplanter parallel ridge or furrow pattern that is commonly observed in acral pustulosis for a long time. This eruption mimicked lentiginous lesion some kinds of lentiginous lesion and it was difficult to diagnose clinically. A 59-year-old female was referred to our hospital with a pigmented macule on her left palm. She had been to the family doctor for treatment of palmoplanter pustulosis by topical steroid ointment application for several years. According to the a history-taking interview, she said that the lesion had b appeared 5 months before referral to our hospital as a tiny pigmented macule and had gradually enlarged during those 5 months (Fig. -

Review of the Genus Mansonella Faust, 1929 Sensu Lato (Nematoda: Onchocercidae), with Descriptions of a New Subgenus and a New Subspecies
Zootaxa 3918 (2): 151–193 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3918.2.1 http://zoobank.org/urn:lsid:zoobank.org:pub:DE65407C-A09E-43E2-8734-F5F5BED82C88 Review of the genus Mansonella Faust, 1929 sensu lato (Nematoda: Onchocercidae), with descriptions of a new subgenus and a new subspecies ODILE BAIN1†, YASEN MUTAFCHIEV2, KERSTIN JUNKER3,8, RICARDO GUERRERO4, CORALIE MARTIN5, EMILIE LEFOULON5 & SHIGEHIKO UNI6,7 1Muséum National d'Histoire Naturelle, Parasitologie comparée, UMR 7205 CNRS, CP52, 61 rue Buffon, 75231 Paris Cedex 05, France 2Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 2 Gagarin Street, 1113 Sofia, Bulgaria E-mail: [email protected] 3ARC-Onderstepoort Veterinary Institute, Private Bag X05, Onderstepoort, 0110, South Africa 4Instituto de Zoología Tropical, Faculdad de Ciencias, Universidad Central de Venezuela, PO Box 47058, 1041A, Caracas, Venezuela. E-mail: [email protected] 5Muséum National d'Histoire Naturelle, Parasitologie comparée, UMR 7245 MCAM, CP52, 61 rue Buffon, 75231 Paris Cedex 05, France E-mail: [email protected], [email protected] 6Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia E-mail: [email protected] 7Department of Parasitology, Graduate School of Medicine, Osaka City University, Abeno-ku, Osaka 545-8585, Japan 8Corresponding author. E-mail: [email protected] †In memory of our colleague Dr Odile Bain, who initiated this study and laid the ground work with her vast knowledge of the filarial worms and detailed morphological studies of the species presented in this paper Table of contents Abstract . -

Zoonotic Abbreviata Caucasica in Wild Chimpanzees (Pan Troglodytes Verus) from Senegal
pathogens Article Zoonotic Abbreviata caucasica in Wild Chimpanzees (Pan troglodytes verus) from Senegal Younes Laidoudi 1,2 , Hacène Medkour 1,2 , Maria Stefania Latrofa 3, Bernard Davoust 1,2, Georges Diatta 2,4,5, Cheikh Sokhna 2,4,5, Amanda Barciela 6 , R. Adriana Hernandez-Aguilar 6,7 , Didier Raoult 1,2, Domenico Otranto 3 and Oleg Mediannikov 1,2,* 1 IRD, AP-HM, Microbes, Evolution, Phylogeny and Infection (MEPHI), IHU Méditerranée Infection, Aix Marseille Univ, 19-21, Bd Jean Moulin, 13005 Marseille, France; [email protected] (Y.L.); [email protected] (H.M.); [email protected] (B.D.); [email protected] (D.R.) 2 IHU Méditerranée Infection, 19-21, Bd Jean Moulin, 13005 Marseille, France; [email protected] (G.D.); [email protected] (C.S.) 3 Department of Veterinary Medicine, University of Bari, 70010 Valenzano, Italy; [email protected] (M.S.L.); [email protected] (D.O.) 4 IRD, SSA, APHM, VITROME, IHU Méditerranée Infection, Aix-Marseille University, 19-21, Bd Jean Moulin, 13005 Marseille, France 5 VITROME, IRD 257, Campus International UCAD-IRD, Hann, Dakar, Senegal 6 Jane Goodall Institute Spain and Senegal, Dindefelo Biological Station, Dindefelo, Kedougou, Senegal; [email protected] (A.B.); [email protected] (R.A.H.-A.) 7 Department of Social Psychology and Quantitative Psychology, Faculty of Psychology, University of Barcelona, Passeig de la Vall d’Hebron 171, 08035 Barcelona, Spain * Correspondence: [email protected]; Tel.: +33-041-373-2401 Received: 19 April 2020; Accepted: 23 June 2020; Published: 27 June 2020 Abstract: Abbreviata caucasica (syn. -

Hutchinson's Melanotic Freckle
Hutchiso’s elaotic freckle Also known as Lentigo maligna What is lentigo maligna? Lentigo maligna is an early form of melanoma. In lentigo maligna the cancer cells are confined to the upper layer of the skin (epidermis). When the cancer cells spread deeper into the skin (to dermis) it is called lentigo maligna melanoma. Lentigo maligna occurs most commonly in sun damaged areas such as the face and neck in fair skinned people over the age of 60. The lesion grows slowly in size over a number of years. Melanoma is a potentially lethal disease and lentigo maligna should be diagnosed and excised as soon as possible. What causes lentigo maligna? The cause of lentigo maligna is sun exposure or solarium use. Factors that predispose a person to developing lentigo maligna or associated condition include: . chronic sun damaged/solar-induced skin damage . fair skin complexion . male gender . a personal history of non melanoma skin cancer and precancerous lesions . older individuals (those between 60 to 80 years are most commonly affected) What does lentigo maligna look like? Lentigo maligna commonly looks like a freckle, age spot, sun spot or brown patch that slowly changes shape and grows in size. The spot may be large in size, irregularly shaped with a smooth surface, and of multiple shades of brown and sometimes other colours. Thickening of part of the lesion, increasing number of colours, ulceration or bleeding can be markers that the lesion is changing into a lentigo maligna melanoma. How is lentigo maligna diagnosed? Lentigo maligna is diagnosed clinically by a dermatologist, sometimes with the help of a dermatoscope (a tool used to magnify and look closely at skin moles). -

Positive Rates of Anti-Acari-Borne Disease Antibodies of Rural Inhabitants in Japan
NOTE Public Health Positive rates of anti-acari-borne disease antibodies of rural inhabitants in Japan Tsutomu TAKEDA1)*, Hiromi FUJITA2), Masumi IWASAKI3), Moe KASAI3), Nanako TATORI4), Tomohiko ENDO5), Yuuji KODERA1,5) and Naoto YAMABATA6) 1)Wildlife Section, Center for Weed and Wildlife Management, Utsunomiya University, 350 Mine, Utsunomiya-shi, Tochigi 321-8505, Japan 2)Mahara Institute of Medical Acarology, 56-3 Aratano, Anan-shi, Tokushima 779-1510, Japan 3)Nikko Yumoto Visitor Center, National Parks Foundation Nikko Branch, Yumoto, Nikko-shi, Tochigi 321-1662, Japan 4)Department of Agriculture, Utsunomiya University, 350 Mine, Utsunomiya-shi, Tochigi 321-8505, Japan 5)United Graduate School of Agricultural Science, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu-shi, Tokyo 183-8509, Japan 6)Institute of Natural and Environmental Sciences, University of Hyogo, Sawano 940, Aogaki-cho, Tanba, Hyogo 669-3842, Japan J. Vet. Med. Sci. ABSTRACT. An assessment of acari (tick and mite) borne diseases was required to support 81(5): 758–763, 2019 development of risk management strategies in rural areas. To achieve this objective, blood samples were mainly collected from rural residents participating in hunting events. Out of 1,152 doi: 10.1292/jvms.18-0572 blood samples, 93 were positive against acari-borne pathogens from 12 prefectures in Japan. Urban areas had a lower rate of positive antibodies, whereas mountainous farming areas had a higher positive antibody prevalence. Residents of mountain areas were bitten by ticks or mites Received: 25 September 2018 significantly more often than urban residents. Resident of mountain areas, including hunters, may Accepted: 9 March 2019 necessary to be educated for prevention of akari-borne infectious diseases. -

Pigmented Contact Dermatitis and Chemical Depigmentation
18_319_334* 05.11.2005 10:30 Uhr Seite 319 Chapter 18 Pigmented Contact Dermatitis 18 and Chemical Depigmentation Hideo Nakayama Contents ca, often occurs without showing any positive mani- 18.1 Hyperpigmentation Associated festations of dermatitis such as marked erythema, with Contact Dermatitis . 319 vesiculation, swelling, papules, rough skin or scaling. 18.1.1 Classification . 319 Therefore, patients may complain only of a pigmen- 18.1.2 Pigmented Contact Dermatitis . 320 tary disorder, even though the disease is entirely the 18.1.2.1 History and Causative Agents . 320 result of allergic contact dermatitis. Hyperpigmenta- 18.1.2.2 Differential Diagnosis . 323 tion caused by incontinentia pigmenti histologica 18.1.2.3 Prevention and Treatment . 323 has often been called a lichenoid reaction, since the 18.1.3 Pigmented Cosmetic Dermatitis . 324 presence of basal liquefaction degeneration, the ac- 18.1.3.1 Signs . 324 cumulation of melanin pigment, and the mononucle- 18.1.3.2 Causative Allergens . 325 ar cell infiltrate in the upper dermis are very similar 18.1.3.3 Treatment . 326 to the histopathological manifestations of lichen pla- 18.1.4 Purpuric Dermatitis . 328 nus. However, compared with typical lichen planus, 18.1.5 “Dirty Neck” of Atopic Eczema . 329 hyperkeratosis is usually milder, hypergranulosis 18.2 Depigmentation from Contact and saw-tooth-shape acanthosis are lacking, hyaline with Chemicals . 330 bodies are hardly seen, and the band-like massive in- 18.2.1 Mechanism of Leukoderma filtration with lymphocytes and histiocytes is lack- due to Chemicals . 330 ing. 18.2.2 Contact Leukoderma Caused Mainly by Contact Sensitization . -

Implementation Manual for the National Epidemiological Surveillance of Infectious Diseases Program
Implementation Manual for the National Epidemiological Surveillance of Infectious Diseases Program Part I. Purpose and Aim The National Epidemiological Surveillance of Infectious Diseases (NESID) Program was started in July 1981 with 18 target diseases. It has been operated with reinforcement and expansion along the way, including the adoption of a computerized online system and an increase in the target diseases to 27 diseases since January 1987. In response to the enactment of the Act on the Prevention of Infectious Disease and Medical Care for Patients with Infectious Diseases (Act No. 114 of 1998; hereinafter referred to as the “Act”) in September 1998 and its enforcement from April 1999, the NESID Program was positioned as a statutory measure. This program will build an appropriate system with cooperation from physicians and other healthcare workers, in order to prevent outbreaks and spread of various infectious diseases by ensuring that measures are taken for the effective and appropriate prevention, diagnosis and treatment of infectious diseases through the accurate monitoring and analysis of information on the occurrences of infectious diseases and through prompt provision and public disclosure of findings from such monitoring and analysis to the general public and healthcare workers, in order to design appropriate measures against infectious diseases by monitoring the detection status of, and identifying the characteristics of, circulating pathogens through collection and analysis of information on the pathogens. Part II. Target Infectious Diseases Target infectious diseases of this surveillance program shall be as follows. 1. Infectious diseases requiring report of all cases (notifiable diseases) Category I Infectious Diseases (1) Ebola hemorrhagic fever, (2) Crimean-Congo hemorrhagic fever, (3) smallpox, (4) South American hemorrhagic fever, (5) plague, (6) Marburg disease, (7) Lassa fever. -

Infectious Diseases Weekly Report Tokyo
Infectious Diseases Weekly Report Tokyo Metropolitan Infectious Disease Surveillance Center 3 June 2021 / Number 21 ( 5/24 ~ 5/30 ) Surveillance System in Tokyo, Japan The infectious diseases which all physicians must report All physicians must report to health centers the incidence of the diseases which are shown at page one. Health centers electronically report the individual cases to Tokyo Metropolitan Infectious Disease Surveillance Center. The infectious diseases required to be reported by the sentinels The numbers of patients who visit the sentinel clinics or hospitals during a week are reported to health centers in Tokyo. And they electronically report the numbers to Tokyo Metropolitan Infectious Disease Surveillance Center. We have about 500 sentinel clinics and hospitals in Tokyo. Tokyo Metropolitan Institute of Public Health TEL:81-3-3363-3213 FAX:81-3-5332-7365 e-mail:[email protected] URL:idsc.tokyo-eiken.go.jp/ Number of patients with the diseases which all physicians must report Tokyo Japan Category Diseases Cum Cum 18th 19th 20th 21st 21st 2021 2021 Ebola hemorrhagic fever Crimean-Congo hemorrhagic fever Smallpox I South American hemorrhagic fever Plague Marburg disease Lassa fever Acute poliomyelitis Tuberculosis 26 32 63 45 883 268 6,125 Diphtheria II Severe Acute Respiratory Syndrome(SARS) Middle East Respiratory Syndrome (MERS) Avian influenza H5N1 Avian influenza H7N9 Cholera Shigellosis 24 III Enterohemorrhagic Escherichia coli infection 22885349483 Typhoid fever Paratyphoid fever Hepatitis E 1441631226 West Nile -

Acacia Flat Mite (Brevipalpus Acadiae Ryke & Meyer, Tenuipalpidae, Acarina): Doringboomplatmyt
Creepie-crawlies and such comprising: Common Names of Insects 1963, indicated as CNI Butterfly List 1959, indicated as BL Some names the sources of which are unknown, and indicated as such Gewone Insekname SKOENLAPPERLYS INSLUITENDE BOSLUISE, MYTE, SAAMGESTEL DEUR DIE AALWURMS EN SPINNEKOPPE LANDBOUTAALKOMITEE Saamgestel deur die MET MEDEWERKING VAN NAVORSINGSINSTITUUT VIR DIE PLANTBESKERMING TAALDIENSBURO Departement van Landbou-tegniese Dienste VAN DIE met medewerking van die DEPARTEMENT VAN ONDERWYS, KUNS EN LANDBOUTAALKOMITEE WETENSKAP van die Taaldiensburo 1959 1963 BUTTERFLY LIST Common Names of Insects COMPILED BY THE INCLUDING TICKS, MITES, EELWORMS AGRICULTURAL TERMINOLOGY AND SPIDERS COMMITTEE Compiled by the IN COLLABORATION WiTH PLANT PROTECTION RESEARCH THE INSTITUTE LANGUAGE SERVICES BUREAU Department of Agricultural Technical Services OF THE in collaboration with the DEPARTMENT OF EDUCATION, ARTS AND AGRICULTURAL TERMINOLOGY SCIENCE COMMITTEE DIE STAATSDRUKKER + PRETORIA + THE of the Language Service Bureau GOVERNMENT PRINTER 1963 1959 Rekenaarmatig en leksikografies herverwerk deur PJ Taljaard e-mail enquiries: [email protected] EXPLANATORY NOTES 1 The list was alphabetised electronically. 2 On the target-language side, ie to the right of the :, synonyms are separated by a comma, e.g.: fission: klowing, splyting The sequence of the translated terms does NOT indicate any preference. Preferred terms are underlined. 3 Where catchwords of similar form are used as different parts of speech and confusion may therefore