Compositions Containing Berberine Or Analogs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Diagnostic Approach to Pruritus
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Air Force Research U.S. Department of Defense 2011 A Diagnostic Approach to Pruritus Brian V. Reamy Christopher W. Bunt Stacy Fletcher Follow this and additional works at: https://digitalcommons.unl.edu/usafresearch This Article is brought to you for free and open access by the U.S. Department of Defense at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in U.S. Air Force Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. A Diagnostic Approach to Pruritus BRIAN V. REAMY, MD, Uniformed Services University of the Health Sciences, Bethesda, Maryland CHRISTOPHER W. BUNT, MAJ, USAF, MC, and STACY FLETCHER, CAPT, USAF, MC Ehrling Bergquist Family Medicine Residency Program, Offutt Air Force Base, Nebraska, and the University of Nebraska Medical Center, Omaha, Nebraska Pruritus can be a symptom of a distinct dermatologic condition or of an occult underlying systemic disease. Of the patients referred to a dermatologist for generalized pruritus with no apparent primary cutaneous cause, 14 to 24 percent have a systemic etiology. In the absence of a primary skin lesion, the review of systems should include evaluation for thyroid disorders, lymphoma, kidney and liver diseases, and diabetes mellitus. Findings suggestive of less seri- ous etiologies include younger age, localized symptoms, acute onset, involvement limited to exposed areas, and a clear association with a sick contact or recent travel. Chronic or general- ized pruritus, older age, and abnormal physical findings should increase concern for underly- ing systemic conditions. -

Photodermatoses Update Knowledge and Treatment of Photodermatoses Discuss Vitamin D Levels in Photodermatoses
Ashley Feneran, DO Jenifer Lloyd, DO University Hospitals Regional Hospitals AMERICAN OSTEOPATHIC COLLEGE OF DERMATOLOGY Objectives Review key points of several photodermatoses Update knowledge and treatment of photodermatoses Discuss vitamin D levels in photodermatoses Types of photodermatoses Immunologically mediated disorders Defective DNA repair disorders Photoaggravated dermatoses Chemical- and drug-induced photosensitivity Types of photodermatoses Immunologically mediated disorders Polymorphous light eruption Actinic prurigo Hydroa vacciniforme Chronic actinic dermatitis Solar urticaria Polymorphous light eruption (PMLE) Most common form of idiopathic photodermatitis Possibly due to delayed-type hypersensitivity reaction to an endogenous cutaneous photo- induced antigen Presents within minutes to hours of UV exposure and lasts several days Pathology Superficial and deep lymphocytic infiltrate Marked papillary dermal edema PMLE Treatment Topical or oral corticosteroids High SPF Restriction of UV exposure Hardening – natural, NBUVB, PUVA Antimalarial PMLE updates Study suggests topical vitamin D analogue used prophylactically may provide therapeutic benefit in PMLE Gruber-Wackernagel A, Bambach FJ, Legat A, et al. Br J Dermatol, 2011. PMLE updates Study seeks to further elucidate the pathogenesis of PMLE Found a decrease in Langerhans cells and an increase in mast cell density in lesional skin Wolf P, Gruber-Wackernagel A, Bambach I, et al. Exp Dermatol, 2014. Actinic prurigo Similar to PMLE Common in native -
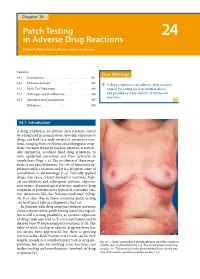
Patch Testing in Adverse Drug Reactions Has Not Always Been Appreciated, but There Is Growing a Interest in This Field
24_401_412 05.11.2005 10:37 Uhr Seite 401 Chapter 24 Patch Testing 24 in Adverse Drug Reactions Derk P.Bruynzeel, Margarida Gonçalo Contents Core Message 24.1 Introduction . 401 24.2 Pathomechanisms . 403 í A drug eruption is an adverse skin reaction 24.3 Patch Test Indications . 404 caused by a drug used in normal doses 24.4 Technique and Test Materials . 406 and presents a wide variety of cutaneous reactions. 24.5 Relevance and Consequences . 407 References . 408 24.1 Introduction A drug eruption is an adverse skin reaction caused by a drug used in normal doses. Systemic exposure to drugs can lead to a wide variety of cutaneous reac- tions, ranging from erythema, maculopapular erup- tions (the most frequent reaction pattern), acrovesic- ular dermatitis, localized fixed drug eruptions, to toxic epidermal necrolysis and from urticaria to anaphylaxis (Figs. 1, 2). The incidence of these erup- tions is not exactly known; 2%–5% of inpatients ex- perience such a reaction and it is a frequent cause of consultation in dermatology [1–3]. Topically applied drugs may cause contact dermatitis reactions. Topi- cal sensitization and subsequent systemic exposure may induce dermatological patterns similar to drug eruptions or patterns more typical of a systemic con- tact dermatitis, like the “baboon syndrome” (Chap. 16). It is clear that, in these situations, patch testing can be of great help as a diagnostic tool [4]. In patients with drug eruptions without previous contact sensitization, patch testing seems less logical, but is still a strong possibility, as systemic exposure of drugs may also lead to T-cell sensitization and to delayed type IV hypersensitivity reactions [5–8]. -

Practical Dermatology Methodical Recommendations
Vitebsk State Medical University Practical Dermatology Methodical recommendations Adaskevich UP, Valles - Kazlouskaya VV, Katina MA VSMU Publishing 2006 616.5 удк-б-1^«адл»-2о -6Sl«Sr83p3»+4£*łp30 А28 Reviewers: professor Myadeletz OD, head of the department of histology, cytology and embryology in VSMU: professor Upatov Gl, head of the department of internal diseases in VSMU Adaskevich IIP, Valles-Kazlouskaya VV, Katina МЛ. A28 Practical dermatology: methodical recommendations / Adaskevich UP, Valles-Kazlouskaya VV, Katina MA. - Vitebsk: VSMU, 2006,- 135 p. Methodical recommendations “Practical dermatology” were designed for the international students and based on the typical program in dermatology. Recommendations include tests, clinical tasks and practical skills in dermatology that arc used as during practical classes as at the examination. УДК 616.5:37.022.=20 ББК 55.83p30+55.81 p30 C Adaskev ich UP, Valles-Ka/.louskaya VV, Katina MA. 2006 OVitebsk State Medical University. 2006 Content 1. Practical skills.......................................................................................................5 > 1.1. Observation of the patient's skin (scheme of the case history).........................5 1.2. The determination of skin moislness, greasiness, dryness and turgor.......... 12 1.3. Dermographism determination.........................................................................12 1.4. A method of the arrangement of dropping and compressive allergic skin tests and their interpretation........................................................................................................ -

DRUG-INDUCED PHOTOSENSITIVITY (Part 1 of 4)
DRUG-INDUCED PHOTOSENSITIVITY (Part 1 of 4) DEFINITION AND CLASSIFICATION Drug-induced photosensitivity: cutaneous adverse events due to exposure to a drug and either ultraviolet (UV) or visible radiation. Reactions can be classified as either photoallergic or phototoxic drug eruptions, though distinguishing between the two reactions can be difficult and usually does not affect management. The following criteria must be met to be considered as a photosensitive drug eruption: • Occurs only in the context of radiation • Drug or one of its metabolites must be present in the skin at the time of exposure to radiation • Drug and/or its metabolites must be able to absorb either visible or UV radiation Photoallergic drug eruption Phototoxic drug eruption Description Immune-mediated mechanism of action. Response is not dose-related. More frequent and result from direct cellular damage. May be dose- Occurs after repeated exposure to the drug dependent. Reaction can be seen with initial exposure to the drug Incidence Low High Pathophysiology Type IV hypersensitivity reaction Direct tissue injury Onset >24hrs <24hrs Clinical appearance Eczematous Exaggerated sunburn reaction with erythema, itching, and burning Localization May spread outside exposed areas Only exposed areas Pigmentary changes Unusual Frequent Histology Epidermal spongiosis, exocytosis of lymphocytes and a perivascular Necrotic keratinocytes, predominantly lymphocytic and neutrophilic inflammatory infiltrate dermal infiltrate DIAGNOSIS Most cases of drug-induced photosensitivity can be diagnosed based on physical examination, detailed clinical history, and knowledge of drug classes typically implicated in photosensitive reactions. Specialized testing is not necessary to make the diagnosis for most patients. However, in cases where there is no prior literature to support a photosensitive reaction to a given drug, or where the diagnosis itself is in question, implementing phototesting, photopatch testing, or rechallenge testing can be useful. -

Studies in Abnormal Human Sensitivity to Light I
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector STUDIES IN ABNORMAL HUMAN SENSITIVITY TO LIGHT I. PRTJRIGO AEST1VALIS, ECZEMA SOLARE AND URTICARIA PHOTOGENICA. REPORT OF 17CASESAND REVIEW OF THE L1TERATTJRE STEPHAN EPSTEIN, M.D.' Marshfield, Wisconsin (Received for publication May 6, 1942) Prurigo aestivalis, eczema solare and urticaria photogenica form a special group among those dermatoses which are considered as caused by light. Rela- tively little is known about them. They have received only short mention in most textbooks; few investigators have concerned themselves with experimental studies. The following is a report of clinical and experimental investigations of these conditions which will be presented in four parts. I. Clinical report. II. Experimental study of light sensitivity. III. Passive transfer in prurigo aestivalis. IV. Photoallergic concept of prurigo aestivalis. I The confusion which exists in regard to diagnosis and classification of these conditions warrants a short review of their clinical diagnosis.2 1. Prurigo aestivalis:Thiscondition, also known as chronic polymorphic light eruption, dermatopathia photogenica, erythema perstans solare, was first described by Hutchinson as summer prurigo in 1888. It is characterized by re- current eruptions of prurigo-like papules and nodules on those parts of the body which are exposed to the sunlight, face, neck, arms, hands, jugular triangle and to some extent, the lower legs (Figs. 1—3). The first symptom is burning and itching of the skin followed by erythema with more or less urticaria-like swelling. Combination with urticaria has been observed. The clinical picture is varied and may change in the same patient. -

COFP Janmar Text
B I O T E R R O R I S M PRINCIPLES AND PRACTICE OF DERMATOLOGY Dr Matthew Ng Joo Ming INTRODUCTION DIAGNOSIS Medical schools and textbooks teach us The diagnosis of skin lesion is made by following dermatology by subjects such as eczema and the same general principle as in any branch of psoriasis. This is useful only for students who are medicine. The process begins by taking a history, new to dermatology. However, it has no use to the followed by a physical examination and if the practicing physician. Patient don’t come to you diagnosis is not made at this stage, further with a diagnosis but they come with a problem of investigations can be carried out. an unknown rash. HISTORY MORPHOLOGY OF SKIN LESION K Duration Characteristics morphology, pattern of K Relationship to physical agents: sun exposure, arrangement and lesions found in a particular occupation, and medication anatomical location can strongly suggest a certain K Puritis dermatological diagnosis. For example, group K Size and colour change vesicles on the penis strongly suggest a diagnosis K Family history of herpes simplex infection. A concise summation K Previous treatment of clinical features, including morphology of skin When describing skin lesion, the following should lesions, pattern of any eruption as well as symptoms be identified in turn: (e.g. itch), is helpful in arriving at a diagnosis. K Site and distribution: symmetrical, Consulting a good picture atlas of skin disease also asymmetrical aids clinical diagnosis. K Erythematous and non-erythematous Lesions can be grouped into four distinct K Surface characteristics: smooth, scaly, warty patterns: linear, annular, group and reticular. -

Drug-Induced Photosensitivity
Acta Dermatovenerol Croat 2016;24(1):55-64 REVIEW Drug-induced Photosensitivity Ewelina Bogumiła Zuba1, Sandra Koronowska1, Agnieszka Osmola- Mańkowska2, Dorota Jenerowicz2 1Student Scientific Group at the Department of Dermatology, Medical University of Poznan, Poznan, Poland; 2Department of Dermatology, Medical University of Poznan, Poznan, Poland Corresponding author: ABSTRACT Ultraviolet radiation is considered the main environmental Ewelina Bogumiła Zuba MD physical hazard to the skin. It is responsible for photoaging, sunburns, carcinogenesis, and photodermatoses, including drug-induced photo- Medical University of Poznan sensitivity. Drug-induced photosensitivity is an abnormal skin reaction 49 Przybyszewskiego St either to sunlight or to artificial light. Drugs may be a cause of photoal- 60-355 Poznan lergic, phototoxic, and photoaggravated dermatitis. There are numerous medications that can be implicated in these types of reactions. Recently, Poland non-steroidal anti-inflammatory drugs have been shown to be a com- [email protected] mon cause of photosensitivity. As both systemic and topical medica- tions may promote photosensitive reactions, it is important to take into Received: November 17, 2014 consideration the potential risk of occurrence such reactions, especially Accepted: January 10, 2016 in people chronically exposed to ultraviolet radiation. KEY WORDS: photoallergic contact dermatitis, photosensitizing agents, phototoxic dermatitis INTRODUCTION Drug-induced photosensitivity is an undesirable Photosensitivity might be associated with differ- effect of topically applied or systemically adminis- ent mechanisms such as phototoxicity, photoallergy, trated pharmaceuticals, followed by exposition to pellagra, pseudoporphyria, lichenoid, and lupus ery- sunlight, mainly ultraviolet A (UVA) or/and ultraviolet thematosus reactions. The most common are pho- B (UVB) radiation as well as visible light. -

100 CASES in Dermatology This Page Intentionally Left Blank 100 CASES in Dermatology
100 CASES in Dermatology This page intentionally left blank 100 CASES in Dermatology Rachael Morris-Jones PhD PCME FRCP Consultant Dermatologist & Honorary Senior Lecturer, King’s College Hospital, London, UK Ann-Marie Powell Consultant Dermatologist, Department of Dermatology, St Thomas’ Hospital, London, UK Emma Benton MB ChB MRCP Post-CCT Clinical Research Fellow, St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Trust, London, UK 100 Cases Series Editor: Professor P John Rees MD FRCP Dean of Medical Undergraduate Education, King’s College London School of Medicine at Guy’s, King’s and St Thomas’ Hospitals, London, UK First published in Great Britain in 2011 by Hodder Arnold, an imprint of Hodder Education, a division of Hachette UK 338 Euston Road, London NW1 3BH http://www.hodderarnold.com © 2011 Rachael Morris-Jones, Ann-Marie Powell and Emma Benton All rights reserved. Apart from any use permitted under UK copyright law, this publication may only be reproduced, stored or transmitted, in any form, or by any means with prior permission in writing of the publishers or in the case of reprographic production in accordance with the terms of licences issued by the Copyright Licensing Agency. In the United Kingdom such licences are issued by the Copyright Licensing Agency: Saffron House, 6–10 Kirby Street, London EC1N 8TS Hachette UK’s policy is to use papers that are natural, renewable and recyclable products and made from wood grown in sustainable forests. The logging and manufacturing processes are expected to conform to the environmental regulations of the country of origin. -

Pattern of Skin Disorders in Hilly District of Pauri Garhwal with Their Dermoscopic Findings: an Epidemiological Study
International Journal of Research in Dermatology Kumari N et al. Int J Res Dermatol. 2021 Sep;7(5):652-657 http://www.ijord.com DOI: https://dx.doi.org/10.18203/issn.2455-4529.IntJResDermatol20213339 Original Research Article Pattern of skin disorders in hilly district of Pauri Garhwal with their dermoscopic findings: an epidemiological study Neeti Kumari, Sunanda Verma*, S. D. S. Rawat, A. K. Mehta, Astha Pant Department of Dermatology, Venereology and Leprosy, Shri Guru Ram Rai Institute of Medical and Health Sciences, Dehradun, Uttarakhand, India Received: 16 July 2021 Revised: 18 August 2021 Accepted: 19 August 2021 *Correspondence: Dr. Sunanda Verma, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: The pattern of skin disorders is largely affected by climate, geography, occupation, socio-economic status, nutrition, genetics and habits of the community. Nowadays, dermoscope is being increasingly used as a non- invasive aid in diagnosis of various skin disorders. Objective of the study was to study the etiology, distribution and dermoscopic findings of various skin disorders in 5 remote villages of hilly district of Pauri Garhwal, Uttarakhand over a period of 1 month (April 2021). Methods: The study was conducted as a weekly OPD in 5 villages of Hilly district of Pauri over a period of 1 month with free consultation and medications. Details of patients, their complaints, clinical diagnosis and dermoscopic findings were recorded. -

Derm World: a Journey Through a "Rash" of Clinical Presentations
Derm World: A Journey Through a "Rash" of Clinical Presentations Rob Danoff, DO, MS, FACOFP, FAAFP Derm World: + A Journey Through a “Rash” of Clinical Presentations Rob Danoff DO, MS, FACOFP, FAAFP OMED 2015 + Cutaneous findings in the Newborn Or, what is this? 1 + What is this? + Cutis Marmorata Mottling of skin Transient phenomena Vascular response to cold with immature nervous system Superficial small blood vessels in the skin dilating (red color) and contracting (pale color) at the same time May persist for months Re-warming usually restores the skin to its normal appearance Occurs in about 50% of infants Generally resolves with increasing age and of no significance for most infants 2 + In the Beginning Proof that babies are delivered by storks + What’s the Diagnosis? 3 + Nevus simplex = Stork bite= Salmon patch Red dilitation of blood vessels often on eyelid, face, or nape of neck (stork bite) They are usually small flat patches of pink or red skin with poorly defined borders These exanthems are very common and occur in over 40% of all newborns The facial patches are sometimes referred to as an “angel's kiss” and tend to fade over the first year of life + Nevus simplex = Stork bite= Salmon patch Often deepen in color with crying, straining with defecation, breath holding or with changes in ambient temperature Not painful or itchy Benign course, reassurance, lighten with age Those on the eyelids and below towards the nose usually disappear by 2 to 3 years of age Salmon patches are rarely detected after age 6 years -

An Update on Chronic Prurigo. Wallengren, Joanna
An update on chronic prurigo. Wallengren, Joanna Published in: Current Medical Litterature: Dermatology 2011 Document Version: Publisher's PDF, also known as Version of record Link to publication Citation for published version (APA): Wallengren, J. (2011). An update on chronic prurigo. In Current Medical Litterature: Dermatology (Vol. 16:4, pp. 89-95). Remedica Publishing. Total number of authors: 1 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 89 Leading Article An Update on Chronic Prurigo Joanna Wallengren, MD, PhD University Hospital, Lund, Sweden CML – Dermatology 2011;16(4):89–95. Submit comments or questions for the authors at www.currentmedicalliterature.com The term prurigo, originating from the being more affected than men.