FECU Thesis Template
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Porting a Window Manager from Xlib to XCB
Porting a Window Manager from Xlib to XCB Arnaud Fontaine (08090091) 16 May 2008 Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.3 or any later version pub- lished by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts and no Back-Cover Texts. A copy of the license is included in the section entitled "GNU Free Documentation License". Contents List of figures i List of listings ii Introduction 1 1 Backgrounds and Motivations 2 2 X Window System (X11) 6 2.1 Introduction . .6 2.2 History . .6 2.3 X Window Protocol . .7 2.3.1 Introduction . .7 2.3.2 Protocol overview . .8 2.3.3 Identifiers of resources . 10 2.3.4 Atoms . 10 2.3.5 Windows . 12 2.3.6 Pixmaps . 14 2.3.7 Events . 14 2.3.8 Keyboard and pointer . 15 2.3.9 Extensions . 17 2.4 X protocol client libraries . 18 2.4.1 Xlib . 18 2.4.1.1 Introduction . 18 2.4.1.2 Data types and functions . 18 2.4.1.3 Pros . 19 2.4.1.4 Cons . 19 2.4.1.5 Example . 20 2.4.2 XCB . 20 2.4.2.1 Introduction . 20 2.4.2.2 Data types and functions . 21 2.4.2.3 xcb-util library . 22 2.4.2.4 Pros . 22 2.4.2.5 Cons . 23 2.4.2.6 Example . 23 2.4.3 Xlib/XCB round-trip performance comparison . -

How-To Gnome-Look Guide
HHOOWW--TTOO Written by David D Lowe GGNNOOMMEE--LLOOOOKK GGUUIIDDEE hen I first joined the harddisk, say, ~/Pictures/Wallpapers. right-clicking on your desktop Ubuntu community, I and selecting the appropriate You may have noticed that gnome- button (you know which one!). Wwas extremely look.org separates wallpapers into impressed with the amount of different categories, according to the customization Ubuntu had to size of the wallpaper in pixels. For Don't let acronyms intimidate offer. People posted impressive the best quality, you want this to you; you don't have to know screenshots, and mentioned the match your screen resolution. If you what the letters stand for to themes they were using. They don't know what your screen know what it is. Basically, GTK is soon led me to gnome-look.org, resolution is, click System > the system GNOME uses to the number one place for GNOME Preferences > Screen Resolution. display things like buttons and visual customization. The However, Ubuntu stretches controls. GNOME is Ubuntu's screenshots there looked just as wallpapers quite nicely if you picked default desktop environment. I impressive, but I was very the wrong size, so you needn't fret will only be dealing with GNOME confused as to what the headings about it. on the sidebar meant, and I had customization here--sorry no idea how to use the files I SVG is a special image format that Kubuntu and Xubuntu folks! downloaded. Hopefully, this guide doesn't use pixels; it uses shapes Gnome-look.org distinguishes will help you learn what I found called vectors, which means you can between two versions of GTK: out the slow way. -

A Simplified Graphics System Based on Direct Rendering Manager System
J. lnf. Commun. Converg. Eng. 16(2): 125-129, Jun. 2018 Regular paper A Simplified Graphics System Based on Direct Rendering Manager System Nakhoon Baek* , Member, KIICE School of Computer Science and Engineering, Kyungpook National University, Daegu 41566, Korea Abstract In the field of computer graphics, rendering speed is one of the most important factors. Contemporary rendering is performed using 3D graphics systems with windowing system support. Since typical graphics systems, including OpenGL and the DirectX library, focus on the variety of graphics rendering features, the rendering process itself consists of many complicated operations. In contrast, early computer systems used direct manipulation of computer graphics hardware, and achieved simple and efficient graphics handling operations. We suggest an alternative method of accelerated 2D and 3D graphics output, based on directly accessing modern GPU hardware using the direct rendering manager (DRM) system. On the basis of this DRM support, we exchange the graphics instructions and graphics data directly, and achieve better performance than full 3D graphics systems. We present a prototype system for providing a set of simple 2D and 3D graphics primitives. Experimental results and their screen shots are included. Index Terms: Direct rendering manager, Efficient handling, Graphics acceleration, Light-weight implementation, Prototype system I. INTRODUCTION Rendering speed is one of the most important factors for 3D graphics application programs. Typical present-day graph- After graphics output devices became publicly available, a ics programs need to be able to handle very large quantities large number of graphics applications were developed for a of graphics data. The larger the data size, and the more sen- broad spectrum of uses including computer animations, com- sitive to the rendering speed, the better the speed-up that can puter games, user experiences, and human-computer inter- be achieved, even for minor aspects of the graphics pipeline. -

Linux Graphics Meets the ARM Ecosystem
Linux Graphics Meets the ARM Ecosystem Jesse Barker <[email protected]> April 12, 2011 Find us here on the Linaro wiki Overview ● The Linux Desktop ● The ARM Linux Desktop ● The Subset Approach ● Examples ● Questions ● What's Next The Desktop Window system ● Display management ● Resource management ● Session management ● Event handling ● Application programming interface Protocol Decode Device Independent X (DIX) DRI EXA DDX User space libdrm evdev DRM KMS Kernel GEM/TTM space Input H/W CPU GPU DC Memory Protocol Encode DRI GLX libdrm libX* Core Application Logic User space DRM KMS Kernel GEM/TTM space GPU DC CPU Memory Toolkits/Frameworks ● Create abstraction layer from the underlying window system. ● Provide uniform look-and-feel across platforms. ● Applications don't have to care which system they are running on. ● New backend to the framework adds a new supported platform for a whole bundle of applications. Bells and Whistles ● OpenGL ● Video ● Audio ● Compositing window managers ● Animation The ARM Desktop What's the difference? ● Most differences are “physical” ● Screen size and resolution ● Unified memory pool ● Power vs. raw performance ● Some API (not necessarily, though) ● Window system interfaces ● Rendering interfaces The Subset Approach ● OpenGL ES 2.0 is explicitly defined as a subset of OpenGL 2.1. ● Both have diverged since the original definition. ● Minimize specialized code (e.g., window system interfaces). The “big-ticket” items ● Immediate mode ● Fixed-function vertex processing ● Fixed-function fragment processing ● EGL vs. GLX Examples ● glmark2 ● cairo-gles ● compiz glmark2 ● Based upon opensource glmark by Ben Smith. ● Uses 3D Studio Max for model content. ● Uses SDL for window system abstraction. -

Beyond Eye Candy
COVER STORY Xgl and Compiz An OpenGL-accelerated desktop with Xgl and Compiz BEYOND EYE CANDY www.sxc.hu A member of Suse’s X11 team delivers an insider’s look at Xgl. agement must work hand in hand, we can expect to see more compositing BY MATTHIAS HOPF window managers in the future with the ability to merge both processes. ac fans were ecstatic when The Render extension adds new basic Another important X server compo- Apple introduced the Quartz primitives for displaying images and nent that desperately needs reworking is MExtreme [1] graphics interface, polygons, along with a new glyph sys- the hardware acceleration architecture, which accelerated desktop effects using tem for enhanced font displays. This which is responsible for efficient hard- 3D hardware. Microsoft’s Windows Vista particularly reflects the fact that the leg- ware representation of graphic com- with its Aero technology looks to close acy graphics commands, called core re- mands. The previous XAA architecture is this gap with the Mac. In the world of quests, no longer meet the demands built around core requests, and is there- Linux, Xgl [2] now provides a compara- placed on modern toolkits such as Qt fore difficult to extend. The architecture ble and even more advanced technology and GTK. All primitives can now be outlived its usefulness and needs replac- that supports similar effects. linked to data in the framebuffer using ing. The most promising alternatives are Xgl is an X Server by David Revemann Porter-Duff operators [3], thus support- EXA and OpenGL. that uses OpenGL to implement graphics ing the rendering of semitransparent sur- EXA is straightforward and easy to im- output. -

Oracle R Enterprise User's Guide, Release 11.2 for Linux, Solaris, AIX, and Windows E26499-05
Oracle® R Enterprise User's Guide Release 11.2 for Linux, Solaris, AIX, and Windows E26499-05 June 2012 Oracle R Enterprise User's Guide, Release 11.2 for Linux, Solaris, AIX, and Windows E26499-05 Copyright © 2012, Oracle and/or its affiliates. All rights reserved. Primary Author: Margaret Taft Contributing Author: Contributor: This software and related documentation are provided under a license agreement containing restrictions on use and disclosure and are protected by intellectual property laws. Except as expressly permitted in your license agreement or allowed by law, you may not use, copy, reproduce, translate, broadcast, modify, license, transmit, distribute, exhibit, perform, publish, or display any part, in any form, or by any means. Reverse engineering, disassembly, or decompilation of this software, unless required by law for interoperability, is prohibited. The information contained herein is subject to change without notice and is not warranted to be error-free. If you find any errors, please report them to us in writing. If this is software or related documentation that is delivered to the U.S. Government or anyone licensing it on behalf of the U.S. Government, the following notice is applicable: U.S. GOVERNMENT RIGHTS Programs, software, databases, and related documentation and technical data delivered to U.S. Government customers are "commercial computer software" or "commercial technical data" pursuant to the applicable Federal Acquisition Regulation and agency-specific supplemental regulations. As such, the use, duplication, disclosure, modification, and adaptation shall be subject to the restrictions and license terms set forth in the applicable Government contract, and, to the extent applicable by the terms of the Government contract, the additional rights set forth in FAR 52.227-19, Commercial Computer Software License (December 2007). -

Drawing with Cairo in GTK
Drawing with Cairo in GTK# http://zetcode.com/gui/gtksharp/drawing/ Home Contents Drawing with Cairo in GTK# In this part of the GTK# programming tutorial, we will do some drawing with the Cairo library. Cairo is a library for creating 2D vector graphics. We can use it to draw our own widgets, charts or various effects or animations. Simple drawing The stroke operation draws the outlines of shapes and the fill operation fills the insides of shapes. Next we will demonstrate these two operations. simpledrawing.cs using Gtk; using Cairo; using System; class SharpApp : Window { public SharpApp() : base("Simple drawing") { SetDefaultSize(230, 150); SetPosition(WindowPosition.Center); DeleteEvent += delegate { Application.Quit(); };; DrawingArea darea = new DrawingArea(); darea.ExposeEvent += OnExpose; Add(darea); ShowAll(); } void OnExpose(object sender, ExposeEventArgs args) { DrawingArea area = (DrawingArea) sender; Cairo.Context cr = Gdk.CairoHelper.Create(area.GdkWindow); cr.LineWidth = 9; cr.SetSourceRGB(0.7, 0.2, 0.0); int width, height; width = Allocation.Width; height = Allocation.Height; cr.Translate(width/2, height/2); cr.Arc(0, 0, (width < height ? width : height) / 2 - 10, 0, 2*Math.PI); cr.StrokePreserve(); cr.SetSourceRGB(0.3, 0.4, 0.6); cr.Fill(); ((IDisposable) cr.Target).Dispose(); ((IDisposable) cr).Dispose(); } public static void Main() { Application.Init(); new SharpApp(); Application.Run(); } } In our example, we will draw a circle and will it with a solid color. 1 di 7 16/12/2014 10:47 Drawing with Cairo in GTK# http://zetcode.com/gui/gtksharp/drawing/ gmcs -pkg:gtk-sharp-2.0 -r:/usr/lib/mono/2.0/Mono.Cairo.dll simple.cs Here is how we compile the example. -

Peerconnection Implementation Experience
Experiences from implementing PeerConnection and related APIs @ Ericsson Labs Background • First implementation of the device element during spring 2010 • No peer-to-peer communication spec at the time • A WebSocket-based, server-relayed solution, ‘MediaStreamTransceiver’ was implemented during summer 2010 • Working ConnectionPeer (i.e. the previous spec) implementation in late 2010 • The current PeerConnection implementation is constantly being improved and updated as the spec changes Target platform • Ubuntu 11.04 • Epiphany web browser • WebKitGTK+ • GStreamer • Libnice WebKitGTK+ • WebKit port to the GTK+ GUI toolkit used by GNOME • Using GLib and GObject • Graphics provided by Cairo, HTTP by libsoup, media playback by GStreamer GStreamer (1) • Pipeline-based open source media framework using GObject • Pipeline elements provided by plugins • A great number of plugins for different codecs, transports, etc. • Fairly easy to create new plugins from existing code from other projects • Simple to work with and provides great functionality, we really like GStreamer GStreamer (2) • Since WebKitGTK+ already uses GStreamer for media playback, we could hook into some of the existing infrastructure • For example, the existing sink element for rendering video in the video element • Thanks to GLib, WebKitGTK+ and GStreamer share the same main event loop Libnice • An open source ICE library based on GLib with GStreamer elements • Simple to use with GStreamer • Since we have only run libnice to libnice we have now idea how interoperable it is PeerConnection -

Linux, Yocto and Fpgas
Embedded Open Source Experts Linux, Yocto and FPGAs Integrating Linux and Yocto builds into different SoCs From a Linux software perspective: ➤ Increased demand for Linux on FPGAs ➤ Many things to mange, both technical and practical ➤ FPGAs with integrated CPU cores – very similar many other SoCs Here are some experiences and observations... © Codiax 2019 ● Page 2 Why use Linux? ➤ De-facto standard ➤ Huge HW support ➤ FOSS ➤ Flexible ➤ Adaptable ➤ Stable ➤ Scalable ➤ Royalty free ➤ Vendor independent ➤ Large community ➤ Long lifetime Why not Linux? ➤ Too big ➤ Real-time requirements ➤ Certification ➤ Boot time ➤ Licensing ➤ Too open? Desktop Shells: Desktop Display server: Display BrailleDisplay Touch-Screen Mouse & Keyboard Wayland Compositor Wayland + development tools = a lot code!of source Linux system example weston, clayton,mutter,KWin evdev libinput GNOME Shell D radeon nouveau lima etna_viv freedreno tegra-re lima nouveau radeon freedreno etna_viv e libwayland-server libwayland-server s Cinnamon k t o kms p Linux kernel, Linux kernel, Plasma 2 w i (Kernel Mode Setting) Mode (Kernel d g Cairo-Dock e t s drm (Direct Rendering Manager) Rendering (Direct drm cache coherent L2-Caches L2-Caches cache coherent CPU &GPU Enlight. DR19 System libraries: System oflibraries): form (in the Toolkits Interface User µClibc Pango glibc glibc main memory possibly adaptations to Wayland/Mir libwayland / COGL libwayland Cairo Cairo (Xr) GTK+ Clutter 2D Application 2D GModule GThread GThread GLib GObject Glib GIO ATK devicedrivers other& modules System -
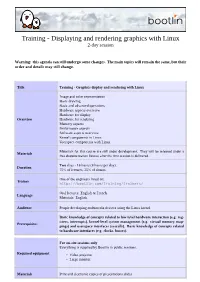
Training - Displaying and Rendering Graphics with Linux 2-Day Session
Training - Displaying and rendering graphics with Linux 2-day session Warning: this agenda can still undergo some changes. The main topics will remain the same, but their order and details may still change. Title Training - Graphics display and rendering with Linux Image and color representation Basic drawing Basic and advanced operations Hardware aspects overview Hardware for display Overview Hardware for rendering Memory aspects Performance aspects Software aspects overview Kernel components in Linux Userspace components with Linux Materials for this course are still under development. They will be released under a Materials free documentation license after the first session is delivered. Two days - 16 hours (8 hours per day). Duration 75% of lectures, 25% of demos. One of the engineers listed on: Trainer https://bootlin.com/training/trainers/ Oral lectures: English or French. Language Materials: English. Audience People developing multimedia devices using the Linux kernel Basic knowledge of concepts related to low-level hardware interaction (e.g. reg- isters, interrupts), kernel-level system management (e.g. virtual memory map- Prerequisites pings) and userspace interfaces (syscalls). Basic knowledge of concepts related to hardware interfaces (e.g. clocks, busses). For on-site sessions only Everything is supplied by Bootlin in public sessions. Required equipment • Video projector • Large monitor Materials Print and electronic copies of presentations slides 1 Day 1 - Morning Lecture - Image and color representation Lecture - Basic drawing and operations • Lines • Pixels and quantization • Circles, ellipses and arcs • Frames, framebuffers and dimensions • Gradients (linear and circular) • Color encoding and depth • Format and colorspace conversion • Colorspaces • Bit blitting • Sub-sampling • Alpha blending • Alpha component • Colorkeying • Pixel formats formalization • Clipping Introducing the basic notions used for representing • Integer scaling color images in graphics. -

New Evolutions in the X Window System
New Evolutions in the X Window System Matthieu Herrb∗ and Matthias Hopf† October 2005 Abstract This paper presents an overview of recent and on-going evolutions in the X window system. First, the state of some features will be presented that are already available for several months in the X server, but not yet widely used in applications. Then some ongoing and future evolutions will be shown: on the short term, the new EXA acceleration framework and the new modular- ized build system. The last part will focus on a longer term project: the new Xgl server architecture, based on the OpenGL technology for both 2D and 3D acceleration. Introduction The X window system celebrated its twentieth birthday last year. After some quick evolution in its early years, its development slowed down during the nineties, be- cause the system had acquired a level of maturity that made it fit most of the needs of the users of graphical work stations. But after all this time, pushed by the com- petition with other systems (Mac OS X and Microsoft Windows) the need for more advanced graphics features triggered new developments. The first part of this paper is going to describe some of these features that are already available (and have been used) for a couple of years but not necessarily known by users of the X window system. A second part will address some on-going work that will be part of the X11R7 release: a new 2D acceleration architecture and the modularization of the source tree. In the third part, a complete redesign of the device dependent layer of the X server, based on OpenGL, will be presented. -

Tizen Overview & Architecture
Tizen Overview & Architecture 삼성전자 정진민 There are many smart devices in mobile market. 2 And, almost as many software platforms for them 3 Many smart devices also appear in non-mobile market 4 User Expectation by it • Before smart device, • The user knew that they were different. • Therefore, the user did not expect anything among them. • Now, • The user is expecting anything among them. • However, They provide different applications and user experiences • Disappointed about inconvenient and incomplete continuation between them. 1 Due to different and proprietary software platform Proprietary platforms 5 Why do they? • Why could not manufacturers provide the same platform for their devices? • The platform has been designed for a specific embedded device. • Manufacturers do not want to share their proprietary platforms. • There is no software platform considering cross category devices as well as fully Open Source. Proprietary platforms 6 What if there is.. • What if there is a standard-based, cross category platform? • The same software can run on many categories of devices with few or no changes • Devices can be connected more easily and provide better convergence services to users • What if the platform is Open Source? • Manufacturers can deploy the platform on their products easily • New features/services can be added without breaking [given the software complies to platform standards] 7 The platform having these two features is ü Standard-based, Cross Category Platform ü Fully Open Source Platform 8 Standard-based, cross category platform