Systematic Review of Immunomodulatory Drugs for the Treatment of People with Multiple Sclerosis: Is There Good Quality Evidence on Evectiveness and Cost?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Practice Guideline: Disease-Modifying Therapies for Adults with Multiple Sclerosis
Practice guideline: Disease-modifying therapies for adults with multiple sclerosis Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology Alexander Rae-Grant, MD1; Gregory S. Day, MD, MSc2; Ruth Ann Marrie, MD, PhD3; Alejandro Rabinstein, MD4; Bruce A.C. Cree, MD, PhD, MAS5; Gary S. Gronseth, MD6; Michael Haboubi, DO7; June Halper, MSN, APN-C, MSCN8; Jonathan P. Hosey, MD9; David E. Jones, MD10; Robert Lisak, MD11; Daniel Pelletier, MD12; Sonja Potrebic, MD, PhD13; Cynthia Sitcov14; Rick Sommers, LMSW15; Julie Stachowiak, PhD16; Thomas S.D. Getchius17; Shannon A. Merillat, MLIS18; Tamara Pringsheim, MD, MSc19 1. Department of Neurology, Cleveland Clinic, OH 2. Department of Neurology, Charles F. and Joanne Knight Alzheimer Disease Research Center, Washington University in St. Louis, MO 3. Departments of Medicine and Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada 4. Department of Neurology, Mayo Clinic, Rochester, MN 5. UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco 6. Department of Neurology, Kansas University Medical Center, Kansas City 7. Department of Neurology, School of Medicine, University of Louisville, KY 8. Consortium of Multiple Sclerosis Centers, Hackensack, NJ 9. Department of Neuroscience, St. Luke’s University Health Network, Bethlehem, PA 10. Department of Neurology, School of Medicine, University of Virginia, Charlottesville 11. Consortium of Multiple Sclerosis Centers, Hackensack, NJ, and Department of Neurology, School of Medicine, Wayne State University, Detroit, MI 12. Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles 13. Neurology Department, Southern California Permanente Medical Group, Kaiser, Los Angeles 14. -

Leflunomide Affect Fertility Or Pregnancy?
Does Leflunomide affect fertility or pregnancy? Leflunomide may cause serious birth defects. Do not take Leflunomide if you are planning to conceive, during pregnancy or when breastfeeding. It is necessary for men and women to use contraception while on National University Hospital Leflunomide.. The drug may remain in the body for up to two years after the last dose. Women Medication Information must not become pregnant and men must not 5 Lower Kent Ridge Road, Singapore 119074 for Patients father children within two years of stopping Website: www.nuh.com.sg Leflunomide; unless medicine is given to get rid LEFLUNOMIDE of it from your body system (consult your doctor). Company Registration No. 198500843R You must not take Leflunomide unless you are on reliable contraception. Pharmacy (Main Building): 6772 5181/5182 Can I have immunisations while on Pharmacy (Kent Ridge Wing): 6772 5184 Leflunomide? Pharmacy (NUH Medical Centre): 6772 8205 Pneumococcal, influenza, hepatitis A and B, tetanus vaccinations are allowed. Avoid immunisations with live vaccines such as polio, varicella (chicken pox), measles, mumps and rubella (MMR). The information provided in this publication is meant purely for educational purposes and may not be used as a substitute for medical diagnosis or treatment. You should seek the advice of your doctor or a qualified healthcare provider before starting any treatment or if you have any questions related to your health, physical fitness or medical conditions. Information is correct at time of printing and subject to revision without prior Are there any alternatives to Leflunomide? notice. Copyright 2014.NationalUniversityHospital Your doctor will advise on the safest and most All Rights reserved. -

Imuran Data Sheet
NEW ZEALAND DATA SHEET 1. IMURAN 25mg, 50mg Imuran tablets (azathioprine 25mg, 50mg) 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 25mg, 50 mg azathioprine BP. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM 25mg: Orange tablet, film coated, round, biconvex, unscored, branded IM2. 50mg: Yellow tablet, film coated, round biconvex, scored, branded IM5. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications IMURAN is used as an immunosuppressant antimetabolite either alone or, more commonly, in combination with other agents (usually corticosteroids) and procedures which influence the immune response. Therapeutic effect may be evident only after weeks or months and can include a steroid- sparing effect, thereby reducing the toxicity associated with high dosage and prolonged usage of corticosteroids. IMURAN, in combination with corticosteroids and/or other immunosuppressive agents and procedures, is indicated to enhance the survival of organ transplants, such as renal transplants, cardiac transplants, and hepatic transplants; and to reduce the corticosteroid requirements of renal transplant recipients. IMURAN is indicated for the treatment of moderate to severe Crohn’s disease in patients in whom corticosteroid therapy is required, in patients who cannot tolerate corticosteroid therapy, or in patients whose disease is refractory to other standard first line therapy. IMURAN, either alone or more usually in combination with corticosteroids and/or other medicines and procedures, has been used with clinical benefit (which may include reduction of dosage or discontinuation of corticosteroids) in a proportion of patients suffering from the following: • severe rheumatoid arthritis; • systemic lupus erythematosus; • dermatomyositis and polymyositis; • auto-immune chronic active hepatitis; • pemphigus vulgaris; • polyarteritis nodosa; • auto-immune haemolytic anaemia; • chronic refractory idiopathic thrombocytopenic purpura; • ulcerative colitis. -

Azathioprine As a Steroid Sparing Agent in Leprosy Type 2 Reactions: Report of Nine Cases
Lepr Rev (2011) 82, 304–309 CASE REPORT Azathioprine as a steroid sparing agent in leprosy Type 2 reactions: Report of nine cases SANDRA MARIA BARBOSA DURA˜ ES*, SIMONE DE ABREU NEVES SALLES**, VERONICA RODRIGUES BOGADO LEITE*** & MORGANA OHIRA GAZZETA**** *Adjunct Professor of Dermatology at Universidade Federal Fluminense, Rio de Janeiro, Brazil **Assistant Professor of Dermatology at Universidade Federal Fluminense, Rio de Janeiro, Brazil ***Graduate Student in Dermatology, Universidade Federal Fluminense, Rio de Janeiro, Brazil ****Medical student at Universidade Federal Fluminense, Rio de Janeiro, Brazil Accepted for publication 06 April 2011 Introduction Glucocorticosteroids (GCS) are the main therapeutic agent used to treat leprosy reactions. Some patients have prolonged reactions and require prolonged therapy with GCS.1,2 Corticosteroid-sparing agents have been used especially in patients with rheumatological diseases to reduce the GCS doses required to control the disease, or as an alternative to GCS, and to reduce side effects. The literature contains few experiences in the use of such GCS sparing agents in leprosy reactions. After methotrexate (whose use is widespread in psoriasis), azathioprine is the immunosuppressant most widely used by dermatologists because of its immunosuppressive effect, which is superior to methotrexate, has less serious side effects than cyclosporine, and a lower cost than that of mycophenolate, tracolimus and sirolimus. These factors guided our predilection for the use of azathioprine over other immunosuppressants since we started using it in 1996, yet there was no publication about its use in leprosy reactions. From 2003 onwards articles began to emerge on the use of azathioprine in leprosy reactions. In the treatment of Type 1 reactions, when followed by prednisolone, it has been found to provide results comparable to those with prednisolone alone,3 thus possibly Correspondence to: Sandra Maria Barbosa Dura˜es, Hospital Universita´rio Antonio Pedro–Universidade Federal Fluminense, Av. -

Immunomodulators
Fact Sheet News from the IBD Help Center IMMUNOMODULATORS Medical treatment for Crohn’s disease and ulcerative colitis has two main goals: achieving remission (control or resolution of inflammation leading to symptom resolution with healing of the inflamed tissue) and then maintaining remission. To accomplish these goals, treatment is aimed at controlling the ongoing inflammation in the intestine—the cause of inflammatory bowel disease (IBD) symptoms. As the name implies, immunomodulators modify the activity of the immune system, in turn, decreasing the inflammatory response. Immunomodulators are most often used in organ transplantation to prevent rejection of the new organ as well as in autoimmune diseases such as rheumatoid arthritis. Since the late 1960s, they have also been used to treat people with IBD, where the normal regulation of the immune system is affected. Immunomodulators, by themselves or with another agent, may be appropriate in the following treatment situations: • Nonresponse or intolerance to aminosalicylates, antibiotics, or corticosteroids • Steroid-dependent disease or frequent need for steroids • Perianal (around the anus) disease that does not respond to antibiotics • Fistulas (abnormal channels between two loops of intestine, or between the intestine and another structure—such as the skin) • To bolster or optimize the effect of a biologic drug and prevent the development of resistance to biologic drugs • To prevent recurrence after surgery Because it can take up to three to six months to see an improvement in symptoms with immunomodulators, steroids may be started at the same time to produce a faster response. Lower doses of the steroid may be utilized in some cases, producing fewer side effects. -

Azathioprine (PDF, 34KB)
Oxford Children’s Hospital Paediatric Gastroenterology Department Azathioprine Information for patients and parents Medicines Information This information leaflet answers common questions parents and patients ask about their medicine. Further information can be found in the information leaflet supplied by the manufacturer, or ask your pharmacist or doctor. page 2 Why have I been started on this medicine? Azathioprine (also known as Imuran®) is often prescribed for patients with chronic active inflammatory bowel disease that requires continuous or repeated courses of corticosteroids. Azathioprine is often referred to as a “steroid sparing agent” or “immunomodulator”. It allows the dose of steroids to be kept to a minimum and eventually stop. Azathioprine is also used in other groups of patients including those with organ transplants, rheumatoid arthritis and psoriasis. How does it work? Azathioprine suppresses inflammation and ‘turns-off’ the activity of the immune system (hence the term “immunomodulator”). Evidence for its effectiveness is stronger in Crohn’s disease but it is widely used in ulcerative colitis. How long does it take to work? Azathioprine acts slowly and can take several weeks to take effect. What dose do I take? The dose of azathioprine is based on weight in children and is around 1-2 mg/kg, once daily. Occasionally, higher doses may be required. How do I take it? Azathioprine comes in tablet form and is available in two different strengths – 50mg and 25mg. The dose should be taken once a day with, or soon after food (it can cause stomach irritation if taken on an empty stomach). We can also order a special liquid form which is 25mg/5ml, but it may take a few days to come in. -

Azathioprine (In Conjunction with Prednisolone) ESCA: for the Treatment of Interstitial Lung Disease AREAS of RESPONSIBILITY for the SHARING of CARE
Effective Shared Care Agreement (ESCA) Azathioprine (in conjunction with prednisolone) ESCA: For the treatment of Interstitial lung disease AREAS OF RESPONSIBILITY FOR THE SHARING OF CARE This shared care agreement outlines suggested ways in which the responsibilities for managing the prescribing of Azathioprine (in conjunction with prednisolone) for Interstitial lung disease can be shared between the specialist and general practitioner (GP). You are invited to participate however, if you do not feel confident to undertake this role, then you are under no obligation to do so. In such an event, the total clinical responsibility for the patient for the diagnosed condition remains with the specialist. Sharing of care assumes communication between the specialist, GP and patient. The intention to share care will be explained to the patient by the specialist initiating treatment. It is important that patients are consulted about treatment and are in agreement with it. Patients with Interstitial lung disease are usually under regular specialist follow-up, which provides an opportunity to discuss drug therapy. The doctor who prescribes the medication legally assumes clinical responsibility for the drug and the consequences of its use. RESPONSIBILITIES and ROLES Specialist responsibilities 1. Confirm the diagnosis of Interstitial lung disease 2. Discuss the potential benefits, treatment side effects, and possible drug interactions with the patient 3. Ask the GP whether he or she is willing to participate in shared care before initiating therapy so that appropriate follow on prescribing arrangements can be made 4. Do baseline monitoring prior to initiation of Azathioprine (in conjunction with prednisolone) 5. Initiate treatment and stabilise dose of Azathioprine (in conjunction with prednisolone) 6. -

The Influence of Immunosuppressants on Non-Melanoma Skin Cancer
The influence of immunosuppressants on non-melanoma skin cancer among patients with systemic lupus erythematosus and primary Sjögren’s syndrome: a nationwide retrospective case-control study in Taiwan H.-W. Tseng1,2, W.-C. Huang3-5, L.-Y. Lu6 1Department of Dermatology, Kaohsiung Veterans General Hospital, Taiwan; 2Institute of Biomedical Sciences, National Sun Yat-Sen University, Taiwan; 3Department of Critical Care Medicine, Kaohsiung Veterans General Hospital, Taiwan; 4School of Medicine, National Yang-Ming University, Taipei, Taiwan; 5Department of Physical Therapy, Fooyin University, Kaohsiung, Taiwan; 6Division of Allergy, Immunology, and Rheumatology, Department of Medicine, Kaohsiung Veterans General Hospital, Taiwan. Abstract Objective To investigate the influence of corticosteroids and hydroxychloroquine on the association with non-melanoma skin cancer (NMSC) among patients with systemic lupus erythematosus (SLE) or primary Sjögren’s syndrome (pSS). Methods This nationwide retrospective case-control study retrieved data from Taiwan National Health Insurance Research Database from 1995-2013. Cases with newly-diagnosed NMSC (n=19,603) and controls without NMSC were matched in a 1:1 ratio according to age, sex, and reference date. SLE, pSS, NMSC, and co-morbidities were determined by ICD-9-CM code. Cumulative drug exposures were defined by cumulative dosages or total defined daily dose (TDDD) of the Anatomical Therapeutic Chemical code of medicines. The analysis used conditional logistic regression and adjusted for age, sex, residential -

IMURAN ® (Azathioprine)
IMURAN ® (azathioprine) 50-mg Scored Tablets PRODUCT INFORMATION Rx only WARNING - MALIGNANCY Chronic immunosuppression with IMURAN, a purine antimetabolite increases risk of malignancy in humans. Reports of malignancy include post-transplant lymphoma and hepatosplenic T-cell lymphoma (HSTCL) in patients with inflammatory bowel disease. Physicians using this drug should be very familiar with this risk as well as with the mutagenic potential to both men and women and with possible hematologic toxicities. Physicians should inform patients of the risk of malignancy with IMURAN. See WARNINGS. DESCRIPTION: IMURAN (azathioprine), an immunosuppressive antimetabolite, is available in tablet form for oral administration. Each scored tablet contains 50 mg azathioprine and the inactive ingredients lactose, magnesium stearate, potato starch, povidone, and stearic acid. Azathioprine is chemically 6-[(1-methyl-4-nitro-1 H-imidazol-5-yl)thio]-1 H-purine. The structural formula of azathioprine is: It is an imidazolyl derivative of 6-mercaptopurine and many of its biological effects are similar to those of the parent compound. Azathioprine is insoluble in water, but may be dissolved with addition of one molar equivalent of alkali. Azathioprine is stable in solution at neutral or acid pH but hydrolysis to mercaptopurine occurs in excess sodium hydroxide (0.1N), especially on warming. Conversion to mercaptopurine also occurs in the presence of sulfhydryl compounds such as cysteine, glutathione, and hydrogen sulfide. CLINICAL PHARMACOLOGY: Azathioprine is well absorbed following oral administration. Maximum serum radioactivity occurs at 1 to 2 hours after oral 35S- azathioprine and decays with a half-life of 5 hours. This is not an estimate of the half-life of azathioprine itself, but is the decay rate for all 35S-containing metabolites of the drug. -

I M M U N O L O G Y Core Notes
II MM MM UU NN OO LL OO GG YY CCOORREE NNOOTTEESS MEDICAL IMMUNOLOGY 544 FALL 2011 Dr. George A. Gutman SCHOOL OF MEDICINE UNIVERSITY OF CALIFORNIA, IRVINE (Copyright) 2011 Regents of the University of California TABLE OF CONTENTS CHAPTER 1 INTRODUCTION...................................................................................... 3 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS ..............................................9 CHAPTER 3 ANTIBODY STRUCTURE I..................................................................17 CHAPTER 4 ANTIBODY STRUCTURE II.................................................................23 CHAPTER 5 COMPLEMENT...................................................................................... 33 CHAPTER 6 ANTIBODY GENETICS, ISOTYPES, ALLOTYPES, IDIOTYPES.....45 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION..................................................................53 CHAPTER 8 GENETIC BASIS OF ANTIBODY DIVERSITY...................................61 CHAPTER 9 IMMUNOGLOBULIN BIOSYNTHESIS ...............................................69 CHAPTER 10 BLOOD GROUPS: ABO AND Rh .........................................................77 CHAPTER 11 CELL-MEDIATED IMMUNITY AND MHC ........................................83 CHAPTER 12 CELL INTERACTIONS IN CELL MEDIATED IMMUNITY ..............91 CHAPTER 13 T-CELL/B-CELL COOPERATION IN HUMORAL IMMUNITY......105 CHAPTER 14 CELL SURFACE MARKERS OF T-CELLS, B-CELLS AND MACROPHAGES...............................................................111 -
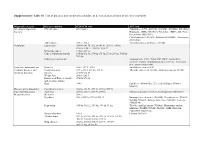
Supplementary Table S1. List of Diseases and Conditions Candidate to Be Tested As Predictors of One-Year Mortality
Supplementary Table S1. List of diseases and conditions candidate to be tested as predictors of one-year mortality Diagnostic category Disease/condition ICD-9 CM code ATC code Infectious and parasitic HIV infection 042.x-044.x Zidovudine (AZT) (J05AF01, J05AR01, J05AR04, J05AR05), diseases Didanosine (DDI) (J05AF02), Zalcitabine (DDC) (J05AF03), Pentamidine (P01CX01), Clarithromycin (J01FA09), Rifabutin (J04AB04), Atovaquone (P01AX06) Tuberculosis 010.x - 018.x Anti-tuberculosis antibiotics (J04AB) Neoplasms Lymphoma 200.00-202.38, 202.50-203.01, 203.8x, 238.6x, 273.3x, V10.71, V10.72, V10.79 Metastatic cancer 196.0x-199.1x Cancer, without metastasis 140.0x-172.9x, 174.0x-175.9x,179.x-195.8x, V10.0x- V10.9x Malignancy medication Antineoplastic (L01), Taxol (C07AB05), Interleukins (L03AC), Colony-stimulating factors (L03AA), Antinausea misc, ondansetron (A04) Endocrine, nutritional and Diabetes 250.x, 357.2, 362.0 Antidiabetic agents (A10) metabolic diseases, and Hypothyroidism 243.x-244.2, 244.8x, 244.9x Thyroid replacement (H03A), Antithyroid agents (H03B) immunity disorders Obesity 278.00-278.01 Weight loss 260.0x-263.9 Disorders of fluid, electrolyte, 276.0x-276.9x and acid-base balance Gout 274.x Colchicine (M04AC01), Uric acid inhibitors (M04AA, M04AB) Diseases of the blood and Coagulation defects 286.0x-286.9x, 287.1x, 287.3x-287.5x blood-forming organs Anaemias 280.0x, 280.1x-281.9x, 285.9x Marrow stimulants (L03AA), Erythropoietin (B03XA01) Mental disorders Dementia 290.x Psychosis 295.x-298.9x, 299.10-299.11 Butyrophenone derivates -

Ethical Use of Off-Label Disease-Modifying Therapies for Multiple Sclerosis
MSJ0010.1177/13524585211030207Multiple Sclerosis JournalJ Laurson-Doube, N Rijke 1030207research-article20212021 MULTIPLE SCLEROSIS MSJ JOURNAL Future Perspectives Multiple Sclerosis Journal Ethical use of off-label disease-modifying 1 –8 https://doi.org/10.1177/13524585211030207DOI: 10.1177/ therapies for multiple sclerosis https://doi.org/10.1177/1352458521103020713524585211030207 © The Author(s), 2021. Joanna Laurson-Doube , Nick Rijke, Anne Helme, Peer Baneke, Brenda Banwell, Article reuse guidelines: Shanthi Viswanathan , Bernhard Hemmer and Bassem Yamout sagepub.com/journals- permissions Abstract Correspondence to: J Laurson-Doube Background: Off-label disease-modifying therapies (DMTs) for multiple sclerosis (MS) are used in at Multiple Sclerosis International Federation, 3rd least 89 countries. There is a need for structured and transparent evidence-based guidelines to support Floor, Skyline House, 200 clinical decision-making, pharmaceutical policies and reimbursement decisions for off-label DMTs. Union Street, London SE1 0LX, UK. Objectives/Results: The authors put forward general principles for the ethical use of off-label DMTs for [email protected] treating MS and a process to assess existing evidence and develop recommendations for their use. Joanna Laurson-Doube Nick Rijke Conclusion: The principles and process are endorsed by the World Federation of Neurology (WFN), Anne Helme American Academy of Neurology (AAN), European Academy of Neurology (EAN), Americas Commit- Peer Baneke Multiple Sclerosis tee for Treatment and Research