The Influence of Preservation Methods of the Color and Other Quality Attributes of Green Beans (Fhaseolus Vulgaris L.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide to Good Hygienic Practices for Packaged Water in Europe
Guide to Good Hygienic Practices for Packaged Water In Europe Guide to Good Hygienic Practices for Packaged Water In Europe GUIDE TO GOOD HYGIENIC PRACTICES FOR PACKAGED WATER IN EUROPE TABLE OF CONTENTS INTRODUCTION 6 ACKNOWLEDGMENTS 6 SCOPE OF THE GUIDE 7 STRUCTURE OF THE GUIDE 7 SECTION 1. GENERAL ASPECTS OF QUALITY & FOOD SAFETY MANAGEMENT 8 1.1. Quality and food safety management systems . 9 1.1.1. Basic principles 9 1.1.2. Documentation 9 1.2. Management responsibility . 10 1.2.1. Management commitment and objectives 10 1.2.2. Quality and food safety policy 10 1.2.3. Quality and food safety management systems planning 10 1.2.4. Responsibility, authority and internal and external communication 10 1.2.5. Management review 11 1.3. Resource management . 12 1.3.1. Provision of resources 12 1.3.2. Human resources 12 1.3.3. Infrastructure and work environment 12 1.4. Control of product quality and safety . 13 1.5. Measurements, analysis and improvement . 14 1.5.1. Monitoring and measurement 14 1.5.2. Analysis of data 14 1.5.3. Continual improvement 14 1.6. Product information and consumer awareness . 15 SECTION 2. PREREQUISITE PROGRAMMES - PRPS 16 2.1. Water resources / Water treatments . 17 2.1.1. Resource development 17 2.1.1.1. General requirements 2.1.1.2. Risk assessment 2.1.2. Resource protection 18 2.1.3. Exploitation of the resource 19 2.1.3.1. Technical requirements 2.1.3.2. Point of abstraction 2.1.3.3. Transfer/piping to the filling operation 2.1.3.4. -

1 Chapter 8130 Department of Revenue Sales and Use Taxes
1 CHAPTER 8130 DEPARTMENT OF REVENUE SALES AND USE TAXES GENERAL PROVISIONS 8130.0110 SCOPE AND INTERPRETATION. 8130.0200 SALE BY TRANSFER OF TITLE. 8130.0400 LEASES. 8130.0500 LICENSE TO USE. 8130.0600 CONSIDERATION. 8130.0700 PRODUCING, FABRICATING, PRINTING, OR PROCESSING OF PROPERTY FURNISHED BY CONSUMER. 8130.0900 ENTERTAINMENT. 8130.1000 LODGING. 8130.1100 UTILITIES AND RESIDENTIAL HEATING FUELS. 8130.1200 SALES OF BUILDING MATERIAL, SUPPLIES, OR EQUIPMENT. 8130.1500 EXEMPTION FOR PROPERTY TAKEN IN TRADE. 8130.1700 DEDUCTIONS ALLOWABLE IN COMPUTING SALES PRICE. 8130.1800 GROSS RECEIPTS DEFINED; METHOD OF REPORTING. 8130.1900 RETAILER AND SELLER. ADMINISTRATION OF SALES AND USE TAXES 8130.2300 IMPOSITION OF SALES TAX. 8130.2500 APPLICATION FOR PERMIT TO MAKE RETAIL SALES. 8130.2700 REINSTATEMENT OF REVOKED PERMITS. 8130.3100 CONTENT AND FORM OF EXEMPTION CERTIFICATE. 8130.3200 NONEXEMPT USE OF PURCHASE OBTAINED WITH EXEMPTION CERTIFICATE. 8130.3300 FUNGIBLE GOODS FOR WHICH EXEMPTION CERTIFICATE GIVEN. 8130.3400 DIRECT PAY AUTHORIZATION PROCEDURE. 8130.3500 MOTOR CARRIERS IN INTERSTATE COMMERCE. 8130.3800 IMPOSITION OF USE TAX. 8130.3900 LIABILITY FOR PAYMENT OF USE TAX. 8130.4000 COLLECTION OF TAX AT TIME OF SALE. 8130.4300 PROPERTY BROUGHT INTO MINNESOTA. 8130.4400 CREDIT AGAINST USE TAX. EXEMPTIONS 8130.4700 PREPARED FOOD, CANDY, AND SOFT DRINKS. 8130.4705 FOOD SOLD WITH EATING UTENSILS. 8130.5300 PETROLEUM PRODUCTS. 8130.5500 AGRICULTURAL AND INDUSTRIAL PRODUCTION. 8130.5550 SPECIAL TOOLING. 8130.5600 PUBLICATIONS. Copyright ©2015 by the Revisor of Statutes, State of Minnesota. All Rights Reserved. 8130.0110 SALES AND USE TAXES 2 8130.5700 SALES TO EXEMPT ENTITIES, THEIR EMPLOYEES, OR AGENTS. -
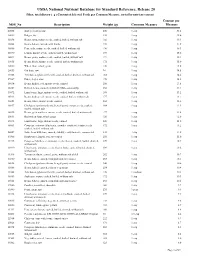
USDA National Nutrient Database for Standard Reference, Release 20
USDA National Nutrient Database for Standard Reference, Release 20 Fiber, total dietary( g ) Content of Selected Foods per Common Measure, sorted by nutrient content Content per NDB_No Description Weight (g) Common Measure Measure 20005 Barley, pearled, raw 200 1 cup 31.2 20012 Bulgur, dry 140 1 cup 25.6 16038 Beans, navy, mature seeds, cooked, boiled, without salt 182 1 cup 19.1 16008 Beans, baked, canned, with franks 259 1 cup 17.9 16086 Peas, split, mature seeds, cooked, boiled, without salt 196 1 cup 16.3 16070 Lentils, mature seeds, cooked, boiled, without salt 198 1 cup 15.6 16043 Beans, pinto, mature seeds, cooked, boiled, without salt 171 1 cup 15.4 16015 Beans, black, mature seeds, cooked, boiled, without salt 172 1 cup 15.0 20080 Wheat flour, whole-grain 120 1 cup 14.6 20033 Oat bran, raw 94 1 cup 14.5 11008 Artichokes, (globe or french), cooked, boiled, drained, without salt 168 1 cup 14.4 09087 Dates, deglet noor 178 1 cup 14.2 16034 Beans, kidney, red, mature seeds, canned 256 1 cup 13.8 16103 Refried beans, canned (includes USDA commodity) 252 1 cup 13.4 16072 Lima beans, large, mature seeds, cooked, boiled, without salt 188 1 cup 13.2 16033 Beans, kidney, red, mature seeds, cooked, boiled, without salt 177 1 cup 13.1 16051 Beans, white, mature seeds, canned 262 1 cup 12.6 16057 Chickpeas (garbanzo beans, bengal gram), mature seeds, cooked, 164 1 cup 12.5 boiled, without salt 16025 Beans, great northern, mature seeds, cooked, boiled, without salt 177 1 cup 12.4 20011 Buckwheat flour, whole-groat 120 1 cup 12.0 16073 Lima -

Initial Report on the Independent Review of the Impacts of the Bottled
Initial report on the Independent review of the impacts of the bottled water industry on groundwater resources in the Northern Rivers region of NSW NSW Chief Scientist & Engineer 1 February 2019 Errata Grammatical and formatting revisions: ‘is’ to ‘has’ (pg. iv); added ‘, which will’ (pg. v); ‘source’ to ‘sources’ (pg. 15); ‘Office of’ to ‘-‘ (pg. 18); removed repetitive word ‘relative’ (pg. 20); ‘5.3.1’ to ‘3.2.1’, ‘5.3.2’ to ‘3.2.2’ and ‘5.3.3’ to ‘3.2.3’ (pg. 33); ‘then’ to ‘Chapter 3’ (pg. 74) pg. 32 – revised ‘licensed for extraction’ to ‘extracted’ pg. 33 – revised ‘extraction rate’ to ‘total water access rights’ pg. 33 – revised ‘estimated’ to ‘calculated’ Chap 3 – ‘planned environmental water (PEW)’ changed to ‘recharge amount reserved for the environment (RRE)’ Addenda pg. 30 Table 3 – added word ‘Supporting‘ to column 3 pg. 33 – added ’80 percent of’ Corrigendum pg. 33 – removed sentence - ‘Where extraction is at or above the LTAAEL allocations can typically be traded within the groundwater source/management area(s).’ www.chiefscientist.nsw.gov.au/reports/independent-review-of-impacts-of-the-bottled-water-industry- on-groundwater-resources-in-the-northern-rivers-region-of-nsw The Hon. Niall Blair MLC Minister for Primary Industries Minister for Regional Water Minister for Trade and Industry 52 Martin Place SYDNEY NSW 2000 1 February 2019 Dear Minister Independent review of the impacts of the bottled water industry on groundwater resources in the Northern Rivers region of NSW In November 2018, you requested that I undertake an independent review of the impacts of the bottled water industry on groundwater resources in the Northern Rivers region of NSW. -

Cantech International May 2020
CANTECH INTERNATIONAL MAY 2020 MAY CANTECH INTERNATIONAL www.cantechonline.com TechTech Can INTERNATIONAL Can MAY2020 CT-COVER MAY20.indd 1 22/04/2020 10:16 Working on the future with Koenig & Bauer MetalPrint. Koenig & Bauer MetalPrint GmbH T +49 711 699 71-0 [email protected] metalprint.koenig-bauer.com Anzeige_Intelligence_CanTech_Apr2020_RZ-3_print.indd 1 17.03.2020 12:55:04 CONTENTS MAY 2020 May 2020 CONTENTS Volume 27, Number 8 22 CANTECH INTERNATIONAL MAY 2020 MAY CANTECH INTERNATIONAL 20 www.cantechonline.com Tech Can INTERNATIONAL Can MAY2020 CT-COVER MAY20.indd 1 22/04/2020 10:16 40 Subscription Information DON’T MISS IT! 17 An annual subscription to CanTech International includes; direct priority mail delivery of 11 issues per year, weekly email newsletter and online access to digital back issues. Print & Digital Issue One Year: £175 UK; £186 Europe; $275 Rest of World Print & Digital Issue Two Years: £302 UK; £320 Europe; $473 Rest of World Digital Issue Only (One year): REGULARS 31 £166/$270 5 Comment To subscribe please email The news as we have it [email protected] or go to www.cantechonline.com/ 7 World News subscribe. Including Twitter Trade Talk 22 COVID-19 10 ways Covid-19 has Send address changes to: 48 New Products changed the can making CanTech International, The latest products available industry forever The Maltings, 57 Bath Street, 40 NEW ZEALAND Gravesend, Kent DA11 0DF, UK. 51 Buyer’s Guide 24 COVID-19 China’s can making industry is Evert van de Weg examines entering a new development 66 The Can Man the consequences for can phase as several of the A sideways look at the world makers due to the Covid-19 country’s leading can © Bell Publishing Ltd 2020. -

Plastic Free
LET'S BREAK UP WITH SINGLE-USE PLASTIC A PRACTICAL HOW-TO GUIDE FOR FOOD VENDORS AND EXHIBITORS MAKING THE SWAP OLD NEW TRADITIONAL COFFEE CUPS THAT SWAP FOR EITHER HOME OR COMMERCIAL HAVE THIN COMPOSTABLE COFFEE CUPS PLASTIC LINING IN WITH A SEPARATE RECYCLABLE LID (RATHER WALLS THAN BIODEGRADABLE). SWAP FOR PAPER BAGS OR START A RETURN PLASTIC BAGS OF SYSTEM ON BAGS. DO NOT USE DEGRADABLE OR ANY SIZE BIODEGRADABLE BAGS. COMPOSTABLE BAGS MAY BREAK IF USED AS CARRY BAG. SWAP TO GLASS OR ALUMINIUM BEVERAGE PLASTIC WATER CONTAINERS. YOU CAN BUY CANNED WATER. BOTTLES AND BEVERAGE ALSO, GUIDE PATRONS TO WATER REFILL STATION CONTAINERS AND BUBBLERS. SWAP TO PAPER STRAWS. PLASTIC STRAWS IF POSSIBLE, OFFER STRAWS ON-REQUEST ONLY. PLASTIC CUTLERY SWAP FOR WOODEN CUTLERY. PLASTIC TAKEAWAY SWAP TO SUGARCANE PULP ALTERNATIVES THAT CONTAINERS AND ARE HOME COMPOSTABLE. STYROFOAM CONTAINERS SWAP TO CARDBOARD THAT IS RECYCLABLE. SINGLE USE PLASTIC MERCHANDISE SWITCH TO SOMETHING THAT CAN BE EITHER RECYCLED EASILY OR COMPOSTED, OR PROVIDE SOMETHING DESIGNED TO LAST. INDIVIDUAL SAUCE, CONFECTIONERY OR SWAP SAUCE PACKETS FOR BULKS DISPENSERS. CONDIMENT WRAPPERS SWAP WRAPPED TREATS FOR HOME-MADE OR PAPER WRAPPED TREATS. THE PROBLEM The impact of plastic pollution on the ocean is being recognised as one of the top environmental challenges of our time. Recent studies estimate that a business-as-usual scenario will lead to more plastic in the ocean than fish, in weight, by 2050. Plastics do not biodegrade, it breaks up into smaller and smaller particles (micro-plastics) that negatively impact the food chain by killing wildlife and marine life. -

Metal Decorator Newsletter
INTERNATIONAL MMETALETAL DDECORATORECORATOR NNEWSLETTEREWSLETTER First Quarter, 2010 ANNOUNCING THE 2010 ANNUAL MEMORIAL SCHOLARSHIP GOLF OUTING & CONVENTION MAY 26—28, 2010 HILTON INDIAN LAKES The time is fast approaching when the metal decorating industry gathers to discuss the state of the art, new techno- logical developments, the latest equipment offerings, regulatory updates, and helpful tips to making your operations and procedures more effective and efficient. Of course, no annual meeting could be a success unless we started on Wednesday with our Memorial Scholarship Golf Outing which sets the tone for great camaraderie and rewarding networking. After a day on the course, the attendees gather in the evening for fun and good food; all in the spirit of raising funds for scholarships for children who have family members who belong to the International Metal Decorators Association. Over the past 5 years we have given more than $23,000 in scholarships; this is a worthy cause. On Thursday we open the day with our membership meeting which is followed by our general session. Metal Packaging, Two Hundred Years of Excellence is our chosen theme for this year which pays tribute to the stability and accomplish- ments of the metal packaging industry. The Convention Committee has developed an agenda which encompasses many aspects of metal decorating in the continuing quest to maintain a scope of excellence in metal packaging. Keynote addresses from industry leaders will inform us on marketing trends and new product developments. Our moti- vational speaker will once again entertain you with thought provoking ideas laden with a humorous side all in the spirit of opening our horizons to become better team players. -

NEWS Page 1/2 It's Aluminum's Time to Shine
NEWS >> https://www.yumda.com/en/news/1162175/ It’s aluminum’s time to shine 30.07.2019 - The bottled water market is in a trans- generational groups. The vilification of plastic bottles formational period. Currently, most mass market represents an opportunity for beverage brands to el- consumers are not especially worried about the en- evate the simple aluminum can. vironmental impact of plastic water bottles; nearly four in 10 consumers would not pay anything more The benefits of aluminum for water packaged in an environmentally friend- Aluminum cans offer compelling environmental ben- ly plastic alternative, according to Mintel research efits; they’re lightweight, durable, have a high recy- on still and sparkling water. However, a growing cling rate, due in part to the financial incentive of conversation around the environmental impact of recycling cans, and are nearly infinitely recyclable. single-use plastic bottles represents an existential Aluminum does come with drawbacks; processing threat to the entire bottled water market. raw aluminum into usable materials is a highly en- ergy intensive process. However, it’s estimated that 75% of all aluminum ever produced in the US is still in use today and reusing aluminum requires only 5% of the energy used compared to processing raw alu- minum. While aluminum is a sustainable packaging solution, consumers are largely in the dark on aluminum’s en- vironmental benefits. Only one in 10 consumers per- ceive aluminum cans as a packaging option that pro- vides a positive environmental impact, according to skeeze/ Pixabay Mintel research on beverage packaging trends. The ubiquity of the can is detrimental to its sustainability symbol image credentials in consumers’ minds as consumers don’t understand aluminum’s environmental benefits and Consumers are beginning to rethink their use of because it is so familiar they don’t view cans as any- plastic water bottles, with Gen Z in particular, exhibit- thing special. -

Options to Reduce Waste Reuse Recycle: Packaged Alternatives
Troublesome item Options to reduce waste Reuse Recycle: Packaged alternatives Plastic takeaway containers Encourage customers by offering a discount for Introduce a reusable container swap program such Recyclable cardboard containers BYO reusable containers as Retub or Returnr, or set up your own! Available from Detpak, The Packaging Place, Alpha Food Packaging and Kent Paper Discourage customers by charging extra for single use containers Recyclable aluminium foil containers for wet foods Available from Confoil and Alpha Food Packaging Recyclable glass jars with metal lids for wet foods Available from Cospak Plastic straws Supply reusable metal straws for dine in Reusable stainless steel straws Recyclable paper straws customers (for dine in) Available from Detpak, The Packaging Place, Alpha Food Packaging and Kent Paper Don’t supply straws for takeaway unless Available from The Clean Collective, Flora & Fauna specifically asked and Zero Store Home compostable wheat stem straws (for takeaway) Business value-add: Sell metal straws Available from Stroh, What a Wheat and Hay Straws Plastic bags Encourage customers by offering a discount for Reusable cloth or woven bags Recyclable paper bags those who bring their own bags Available from The Clean Collective, Flora & Fauna Recyclable paper satchel bags Discourage customers by refusing to supply and Zero Store plastic bags at all Available from Detpak, The Packaging Place, Alpha Food Packaging and Kent Paper Business value-add: Sell reusable cloth or woven bags Tableware Supply reusable metal -

First High Street Chain to Remove All Plastic Bottles from Stores, Replacing with Canned Water Submitted By: KTRPR Wednesday, 15 January 2020
First High Street Chain To Remove All Plastic Bottles From Stores, Replacing With Canned Water Submitted by: KTRPR Wednesday, 15 January 2020 Innovative Italian-to-Go brand, Coco di Mama, has made the decision this January to completely remove all plastic water bottles from its shelves. The new initiative will save 180,000* plastic bottles per year. Coco di Mama will be offering free water refills in all of their stores and also introducing an infinitely recyclable alternative to plastic bottles, with resealable canned water from CanO Water from £1. CanO Water is infinitely recyclable and made from an average of 60% recycled content, compared to plastic water bottles which are made from 50% recycled content. It can be recycled and back on the shelves in around six weeks. Launching 15th January 2020 in all Coco di Mama stores in London New CanO Water will be available to buy in multiple sizes of still and sparkling variants, in all 28 Coco di Mama stores across London for £1 – (see locations and opening times via www.cocodimama.co.uk). Customers can also get free tap water refills in all stores with their own reusable water bottle, or glasses are available for non-takeaway customers. Cans VS Plastic - Why are cans better than plastic bottles? Since plastic bottles are so thin, they can’t be recycled into more plastic bottles. Instead, their fibres are used in things like carpeting, clothing and sleeping bags. Aluminium cans are infinitely recyclable, once recycled they will be turned into new aluminium cans and come back on the shelf in as little as 60 days. -

On the Questionable Appeal of Glossy/Shiny Food Packaging
foods Review On the Questionable Appeal of Glossy/Shiny Food Packaging Charles Spence Department of Experimental Psychology, Anna Watts Building, University of Oxford, Oxford OX2 6BW, UK; [email protected] Abstract: Those stimuli that have a shiny/glossy visual appearance are typically rated as both attrac- tive and attention capturing. Indeed, for millennia, shiny precious metals and glossy lacquerware have been used to enhance the presentation, and thus the perception, of food and drink. As such, one might have expected that adding a shiny/glossy appearance/finish to the outer packaging of food and beverage products would also be desirable. However, the latest research appears to show that many consumers have internalised an association between glossy packaging and greasy (or unhealthy) food products, while matte packaging tends to be associated with those foods that are more natural instead. Furthermore, it turns out that many consumers do not necessarily appreciate the attempt to capture their attention that glossy packaging so often affords. At the same time, it is important to recognise that somewhat different associations may apply in the case of inner versus outer food and beverage packaging. Shiny metallic (inner) packaging may well prime (rightly or wrongly) concerns about sustainability amongst consumers. Given the research that has been published in recent years, food and beverage manufacturers/marketers should think very carefully about whether or not to introduce such shiny/glossy finishes to their packaging. Keywords: packaging; glossy; shiny; material properties; inner; outer; learned associations; evolu- tionary explanations Citation: Spence, C. On the Questionable Appeal of Glossy/Shiny Food Packaging. -

Amounts of Fiber in Common Foods
Amounts of Fiber in Common Foods Common Fiber in Ingredient Measure Grams Barley, pearled, raw 1 cup 31.2 Bulgur, dry 1 cup 25.6 Beans, navy, mature seeds, cooked, boiled, without salt 1 cup 19.1 Beans, baked, canned, with franks 1 cup 17.9 Beans, kidney, red, mature seeds, canned 1 cup 16.4 Peas, split, mature seeds, cooked, boiled, without salt 1 cup 16.3 Lentils, mature seeds, cooked, boiled, without salt 1 cup 15.6 Beans, pinto, mature seeds, cooked, boiled, without salt 1 cup 15.4 Beans, black, mature seeds, cooked, boiled, without salt 1 cup 15.0 Wheat flour, whole-grain 1 cup 14.6 Oat bran, raw 1 cup 14.5 Dates, deglet noor 1 cup 14.2 Refried beans, canned (includes USDA commodity) 1 cup 13.4 Lima beans, large, mature seeds, cooked, boiled, without salt 1 cup 13.2 Beans, kidney, red, mature seeds, cooked, boiled, without salt 1 cup 13.1 Beans, white, mature seeds, canned 1 cup 12.6 Chickpeas (garbanzo beans, bengal gram), mature seeds, cooked, boiled, 1 cup 12.5 without salt Beans, great northern, mature seeds, cooked, boiled, without salt 1 cup 12.4 Buckwheat flour, whole-groat 1 cup 12.0 Tomato products, canned, paste, without salt added 1 cup 11.8 Lima beans, large, mature seeds, canned 1 cup 11.6 Cowpeas, common (blackeyes, crowder, southern), mature seeds, cooked, 1 cup 11.2 boiled, without salt Soup, bean with ham, canned, chunky, ready-to-serve, commercial 1 cup 11.2 Raspberries, frozen, red, sweetened 1 cup 11.0 Cowpeas (blackeyes), immature seeds, frozen, cooked, boiled, drained, without 1 cup 10.9 salt Lima beans, immature