Histologic Analysis of Clinically Healthy Human Gingiva in Patients with Altered Passive Eruption
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Study Minimally Invasive Treatment of Infrabony Periodontal Defects Using Dual-Wavelength Laser Therapy
Hindawi Publishing Corporation International Scholarly Research Notices Volume 2016, Article ID 7175919, 9 pages http://dx.doi.org/10.1155/2016/7175919 Clinical Study Minimally Invasive Treatment of Infrabony Periodontal Defects Using Dual-Wavelength Laser Therapy Rana Al-Falaki,1 Francis J. Hughes,2 and Reena Wadia2 1 Al-FaPerio Clinic, 48AQueensRoad,BuckhurstHill,EssexIG95BY, UK 2Department of Periodontology, King’s College London Dental Institute, Floor 21,TowerWingGuysHospital,LondonSE19RT, UK Correspondence should be addressed to Rana Al-Falaki; [email protected] Received 23 January 2016;Revised15 April 2016;Accepted26 April 2016 Academic Editor: Jiiang H. Jeng Copyright © 2016 Rana Al-Falaki et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Introduction. Surgical management of infrabony defects is an invasive procedure, frequently requiring the use of adjunctive material such as grafs or biologics, which is time-consuming and associated with expense and morbidity to the patient. Lasers in periodontal regeneration have been reported in the literature, with each wavelength having potential benefts through diferent laser-tissue interactions. Te purpose of this case series was to assess the efcacy of a new dual-wavelength protocol in the management of infrabony defects. Materials and Methods. 32 defects (one in each patient) were treated using ultrasonic debridement, followed by fapless application of Erbium, Chromium:Yttrium, Scandium, Gallium, Garnet (Er,Cr:YSGG) laser (wavelength 2780 nm), and fnal application of diode laser (wavelength 940 nm). Pocket depths (PD) were measured afer 6 months and repeat radiographs taken afer one year. -

DENTIN HYPERSENSITIVITY: Consensus-Based Recommendations for the Diagnosis & Management of Dentin Hypersensitivity
October 2008 | Volume 4, Number 9 (Special Issue) DENTIN HYPERSENSITIVITY: Consensus-Based Recommendations for the Diagnosis & Management of Dentin Hypersensitivity A Supplement to InsideDentistry® Published by AEGISPublications,LLC © 2008 PUBLISHER Inside Dentistry® and De ntin Hypersensitivity: Consensus-Based Recommendations AEGIS Publications, LLC for the Diagnosis & Management of Dentin Hypersensitivity are published by AEGIS Publications, LLC. EDITORS Lisa Neuman Copyright © 2008 by AEGIS Publications, LLC. Justin Romano All rights reserved under United States, International and Pan-American Copyright Conventions. No part of this publication may be reproduced, stored in a PRODUCTION/DESIGN Claire Novo retrieval system or transmitted in any form or by any means without prior written permission from the publisher. The views and opinions expressed in the articles appearing in this publication are those of the author(s) and do not necessarily reflect the views or opinions of the editors, the editorial board, or the publisher. As a matter of policy, the editors, the editorial board, the publisher, and the university affiliate do not endorse any prod- ucts, medical techniques, or diagnoses, and publication of any material in this jour- nal should not be construed as such an endorsement. PHOTOCOPY PERMISSIONS POLICY: This publication is registered with Copyright Clearance Center (CCC), Inc., 222 Rosewood Drive, Danvers, MA 01923. Permission is granted for photocopying of specified articles provided the base fee is paid directly to CCC. WARNING: Reading this supplement, Dentin Hypersensitivity: Consensus-Based Recommendations for the Diagnosis & Management of Dentin Hypersensitivity PRESIDENT / CEO does not necessarily qualify you to integrate new techniques or procedures into your practice. AEGIS Publications expects its readers to rely on their judgment Daniel W. -

Epidemiology and Indices of Gingival and Periodontal Disease Dr
PEDIATRIC DENTISTRY/Copyright ° 1981 by The American Academy of Pedodontics Vol. 3, Special Issue Epidemiology and indices of gingival and periodontal disease Dr. Poulsen Sven Poulsen, Dr Odont Abstract Validity of an index indicates to what extent the This paper reviews some of the commonly used indices index measures what it is intended to measure. Deter- for measurement of gingivitis and periodontal disease. mination of validity is dependent on the availability Periodontal disease should be measured using loss of of a so-called validating criterion. attachment, not pocket depth. The reliability of several of Pocket depth may not reflect loss of periodontal the indices has been tested. Calibration and training of attachment as a sign of periodontal disease. This is be- examiners seems to be an absolute requirement for a cause gingival swelling will increase the distance from satisfactory inter-examiner reliability. Gingival and periodontal disease is much more severe in several the gingival margin to the bottom of the clinical populations in the Far East than in Europe and North pocket (pseudo-pockets). Thus, depth of the periodon- America, and gingivitis seems to increase with age resulting tal pocket may not be a valid measurement for perio- in loss of periodontal attachment in approximately 40% of dontal disease. 15-year-old children. Apart from the validity and reliability of an index, important factors such as the purpose of the study, Introduction the level of disease in the population, the conditions under which the examinations are going to be per- Epidemiological data form the basis for planning formed etc., will have to enter into choice of an index. -

Clinical Outcome of a New Surgical Technique for the Treatment of Peri-Implant Dehiscence in the Esthetic Area. a Case Report
applied sciences Case Report Clinical Outcome of a New Surgical Technique for the Treatment of Peri-Implant Dehiscence in the Esthetic Area. A Case Report Norberto Quispe-López 1 , Carmen García-Faria 2, Jesús Mena-Álvarez 2,* , Yasmina Guadilla 1, Pablo Garrido Martínez 3,4 and Javier Montero 1 1 Department of Surgery, Faculty of Medicine, University of Salamanca, 37008 Salamanca, Spain; [email protected] (N.Q.-L.); [email protected] (Y.G.); [email protected] (J.M.) 2 Faculty of Health Sciences, Alfonso X el Sabio University, 28703 Madrid, Spain; [email protected] 3 Department of Prosthesis, Faculty of Dentistry, Universidad Alfonso X el Sabio, 28703 Madrid, Spain; [email protected] 4 Department of Oral and Maxillofacial Surgery, Hospital La Luz, 28003 Madrid, Spain * Correspondence: [email protected] Abstract: This study describes the clinical and esthetic outcome of n apical surgical treatment on peri-implant soft tissue dehiscence in an implant with a poor prognosis in the esthetic area. The patient presented a compromised situation of clinical attachment loss both in the 1.2 implant and in the adjacent teeth. A biphasic approach consisted firstly of a connective tissue graft accessed by apical and then, 11 months later, a palatal flap technique plus a connective tissue graft. After 20 months of Citation: Quispe-López, N.; healing, surgical approaches without vertical releasing incisions showed a gain in recession reduction García-Faria, C.; Mena-Álvarez, J.; over the implant ranging from 0.3 to 2.7 mm (CI 95%), in addition to a gain in width (2 mm) and Guadilla, Y.; Garrido Martínez, P.; thickness (2.3 mm) of the keratinized mucosa. -

04-207 Gingival Flap Procedure and Apically Positioned
Dental Policy Subject: Gingival Flap Procedure and Apically Positioned Flap Guideline #: 04-207 Publish Date: 03/27/2018 Status: New Last Review Date: 03/12//2018 Description This document addresses the Gingival Flap Procedure, including root planing, and Apically Positioned Flap. Note: Please refer to the following documents for additional information concerning related topics: Scaling and Root Planing (04-301) Periodontal Maintenance (04-901) Mucogingival Surgery and Soft Tissue Grafting (04-204) Biological Materials to Aid Soft and Hard Tissue Grafting (04 Clinical Policy-01 Teeth with Guarded or Poor Prognosis Indications The gingival flap procedure or apically positioned flap are considered appropriate for the treatment of mild to severe periodontal disease when non-surgical methods such as scaling and root planing have been unsuccessful in removal of below the gum deposits of plaque (biofilm) and calculus and where, due to supra-bony pocket depths osseous recontouring and bone grafting are not required. As it applies to appropriateness of care, dental services must be: provided by a Dentist, exercising prudent clinical judgment provided to a patient for the purpose of evaluating, diagnosing and/or treating a dental injury or disease or its symptoms in accordance with the generally accepted standards of dental practice which means: o standards that are based on credible scientific evidence published in peer-reviewed, dental literature generally recognized by the practicing dental community o specialty society recommendations/criteria o any other relevant factors clinically appropriate, in terms of type, frequency and extent considered effective for the patient's dental injury or disease not primarily performed for the convenience of the patient or Dentist not more costly than an alternative service. -

Correlation of Width of Attached Gingiva on Oral Hygiene Maintenance and Gingival Health
Research Article J Nepal Soc Perio Oral Implantol. 2020;4(7):5-9 Correlation of Width of Attached Gingiva on Oral Hygiene Maintenance and Gingival Health Dr. Shaili Pradhan,1 Dr. Benju Shrestha1 1Department of Dental Surgery, National Academy of Medical Sciences, Bir Hospital, Kathmandu, Nepal. ABSTRACT Introduction: Attached gingiva aids in increased resistance to external injury and contribute in stabilisation of gingival margin against frictional forces as well as dissipates physiological forces exerted by the muscular fibers of the alveolar mucosa on gingival tissues. Objective: To assess width of attached gingiva in adults and correlate with oral hygiene maintenance and gingival inflammation. Methods: A cross-sectional study was conducted in patients aged 20-40 years visiting dental OPD with healthy periodontium. Plaque index (PI) and Gingival index (GI) were recorded. Mucogingival junction was determined by visual and functional method. Keratinised gingiva width (KGW) and probing pocket depth (PPD) was recorded and attached gingiva width (AGW) was calculated as (KGW–PPD). Results: Total 85 patients (43 males and 42 females) enrolled in this study. Among total, 48.23% had AGW<1 mm. AGW <1 mm most commonly was found in mandibular first premolar, highest mean AGW was found in maxillary incisors. The mean GI and PI values for AGW<1 mm were found to be higher than those for AGW≥ 1 mm. However, result did not show any significant relation between AGW and severity of gingival inflammation (P value 0.608) and plaque control (P value 0.297). Conclusion: The correlation between attached gingiva width and severity of gingival inflammation and plaque index was not significant statistically. -
The Relationship Between the Width of Keratinized Gingiva and Gingival Health By
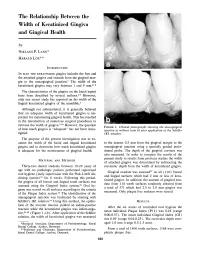
The Relationship Between the Width of Keratinized Gingiva and Gingival Health by NIKLAUS P. LANG* HARALD LÖE** INTRODUCTION IN MAN THE KERATINIZED gingiva includes the free and the attached gingiva and extends from the gingival mar gin to the mucogingival junction.1 The width of the keratinized gingiva may vary between 1 and 9 mm.2, 3 The characteristics of the gingiva on the facial aspect have been described by several authors.17 However, only one recent study has reported on the width of the lingual keratinized gingiva of the mandible.7 Although not substantiated, it is generally believed that an adequate width of keratinized gingiva is im portant for maintaining gingival health. This has resulted in the introduction of numerous surgical procedures to increase the width of gingiva.830 However, the question FIGURE 1. Clinical photographs showing the mucogingival of how much gingiva is "adequate" has not been inves junction a) without stain b) after application of the Schiller tigated. IKI solution. The purpose of the present investigation was to ex amine the width of the facial and lingual keratinized to the nearest 0.5 mm from the gingival margin to the gingiva and to determine how much keratinized gingiva mucogingival junction using a specially graded perio is adequate for the maintenance of gingival health. dontal probe. The depth of the gingival crevices was also measured. In order to compare the results of the present study to results from previous studies the width MATERIAL AND METHODS of attached gingiva was determined by subtracting the Thirty-two dental students between 19-29 years of crevicular depth from the width of keratinized gingiva. -

A Panoramic View of Junctional Epithelium and Biologic Width Around Teeth and Implant
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 16, Issue 9 Ver. IX (September. 2017), PP 61-70 www.iosrjournals.org A Panoramic View of Junctional Epithelium And Biologic Width Around Teeth And Implant *Dr. Jaimini Patel1, Dr. Jasuma Rai2,Dr. Deepak Dave3,Dr. Nidhi Shah4, Dr. Shraddha Shah5 1,2,3,4,5,(Department of Periodontology/ K. M. Shah Dental College and Hospital/ Sumandeep Vidyapeeth, India) Corresponding Author: *Dr. Jaimini Patel Abstract : Junctional epithelium is the most dynamic feature of the periodontal tissues as it not only plays an important role in health but also displays various characteristic changes in disease. The biologic width around tooth and implants is also an important consideration from treatment point of view. In the following review we have discussed the importance of junctional epithelium and biologic width around teeth and implant and the factors that influence the peri-implant biologic width. Keywords : Biologic Width, Junctional Epithelium, Implant ----------------------------------------------------------------------------------------------------------------------------- ---------- Date of Submission: 29 -07-2017 Date of acceptance: 09-09-2017 -------------------------------------------------------------------------------------------------------------------------------------- I. Introduction Teeth are trans-mucosal organs. This is a unique association in the human body where a hard tissue emerges through the soft tissue. Epithelia exhibit considerable differences in their histology, thickness and differentiation suitable for the functional demands of their location.1 The gingival epithelium around a tooth is divided into three functional compartments– outer, sulcular, and junctional epithelium. The outer epithelium extends from the mucogingival junction to the gingival margin where crevicular/sulcular epithelium lines the sulcus. At the base of the sulcus connection between gingiva and tooth is mediated with junctional epithelium. -

Dental Hygiene Clinic Procedure and Policy Manual
Dental Hygiene Clinic Policy and Procedure Manual Ferris State University College of Health Professions Dental Hygiene Program Written and Edited by Annette U. Jackson, RDH, BS, MS (c) In Collaboration with the Dental Hygiene Faculty and Staff Reviewed and Updated 2019 DENTAL CLINIC POLICY AND PROCEDURES MANUAL DENTAL HYGIENE PROGRAM DENTAL CLINIC The intent of this manual is to provide guidelines to students, faculty, and staff concerning their expectations and obligations associated with participation in the Ferris Dental Hygiene clinic. CLINIC PURPOSE The dental hygiene clinic serves as the location for dental hygiene students to receive their pre-clinic and clinical experience in preparation to become a registered dental hygienist. In general, the clinic also serves as the location for the general public to receive dental hygiene care, as they serve as patients for dental hygiene students. As this facility provides patient treatment, it must be recognized that, during the time patients are being treated, all efforts must be directed toward safe, appropriate patient treatment and appropriate student supervision. Only students who are scheduled to treat patients should be present in clinic unless appropriately authorized. Non-clinic related business should not be occurring during scheduled clinic times. Clinic instructors are responsible for supervising the students and patients who have been assigned to them during a clinic session. Students (not scheduled in clinic), who need to speak to a clinic instructor, should make arrangements with the instructor to do so during the instructor’s office hour or other mutually agreeable time, rather than during the instructor’s clinic assignment. Neither students nor instructors should be leaving their assigned clinic to conduct non- related business unless an emergency develops, or if follow up with a patient’s physician, pharmacy, etc., needs to be done. -

Pro-Argin, a Breakthrough Technology Based Upon Arginine
American Journal of Dentistry, Vol. 22, Special Issue A, March, 2009 A, March, 22, Special Issue Vol. American Journal of Dentistry, Vol. 22, Special Issue A, March, 2009 - p. 1A - 24A Introducing Pro-Argin™ A Breakthrough Technology Based upon Arginine and Calcium for In-Office Treatment of Dentin Hypersensitivity _______________________________________________________________________________________________________________________________________________________________ Editorial _______________________________________________________________________________________________________________________________________________________________ Dentin hypersensitivity: Beneficial effects of an arginine-calcium carbonate desensitizing paste Dentin hypersensitivity is a common occurrence diately after dental scaling procedures and its and is often a chief concern among patients. The sustained relief over 4 weeks. Another paper pre- pain associated with dentin hypersensitivity is sents the results of a double-blind, stratified, caused by some type of external stimulus and the randomized clinical study showing the successful sensitivity can range in its intensity from patient to desensitizing effect of the 8% arginine-calcium patient. The successful management of dentin carbonate paste tested, when applied as a pre- hypersensitivity is often very challenging for the procedure to professional dental cleaning. dental professional. The cause of the pain and the This Special Issue also includes a study con- description of the discomfort reported by -

Cervical Restorations Useful When Assessing Gingival and Periodontal Health
http://dx.doi.org/10.17159/2519-0105/2018/v73no10a9 INDUSTRY NEWS < 633 Cervical restorations useful when assessing gingival and periodontal health SADJ November 2018, Vol. 73 No. 10 p633 - p634 A Volchansky “Once a Periodontal Patient, always a Periodontal Patient”. ACRONYM This comment could be describing the affliction of Chronic CEJ: Cemento-enamel-junction Periodontitis or Refractory Periodontitis which refer to the periodontal status of patients who require monitoring over extended periods and who demonstrate severe attach- ment loss (derived from Parameters of Care: Journal of Periodontology, 20001). The progression of periodontal diseases is assessed by the extent of gingival recession, the severity of clinical attachment loss, and the probing depths of pockets. Periodontal disease is generally described as a slow and continually progressive condition.2 It may well be important that a fixed reference point is available to ensure repeatability when these measurements are recorded. The landmark habitually used is the cemento-enamel -junction (CEJ). The normal gingival margin position is 0.5 – 2.0 mm coronal to the CEJ. Gingival recession is defined as the increase in the location of the gingival margin apical to the CEJ.4 Figure 1. Cervical restorations on 13; 14, obscuring the CEJ. A cervical restoration is one that is placed adjacent There are three options, supragingival, equigingival and to the CEJ or the gingival margin (G V Black (19023). subgingival. Much has been written about the importance The margins of such a restoration are clearly visible of the restorative margin, its location, the materials adjacent to the gingival attachment and the location may and the contours of any restoration in relation to peri- approximate the cervical line / cemento-enamel junction odontal health.5,6 of the tooth. -

Diagnosis Questions and Answers
1.0 DIAGNOSIS – 6 QUESTIONS 1. Where is the narrowest band of attached gingiva found? 1. Lingual surfaces of maxillary incisors and facial surfaces of maxillary first molars 2. Facial surfaces of mandibular second premolars and lingual of canines 3. Facial surfaces of mandibular canines and first premolars and lingual of mandibular incisors* 4. None of the above 2. All these types of tissue have keratinized epithelium EXCEPT 1. Hard palate 2. Gingival col* 3. Attached gingiva 4. Free gingiva 16. Which group of principal fibers of the periodontal ligament run perpendicular from the alveolar bone to the cementum and resist lateral forces? 1. Alveolar crest 2. Horizontal crest* 3. Oblique 4. Apical 5. Interradicular 33. The width of attached gingiva varies considerably with the greatest amount being present in the maxillary incisor region; the least amount is in the mandibular premolar region. 1. Both statements are TRUE* 39. The alveolar process forms and supports the sockets of the teeth and consists of two parts, the alveolar bone proper and the supporting alveolar bone; ostectomy is defined as removal of the alveolar bone proper. 1. Both statements are TRUE* 40. Which structure is the inner layer of cells of the junctional epithelium and attaches the gingiva to the tooth? 1. Mucogingival junction 2. Free gingival groove 3. Epithelial attachment * 4. Tonofilaments 1 49. All of the following are part of the marginal (free) gingiva EXCEPT: 1. Gingival margin 2. Free gingival groove 3. Mucogingival junction* 4. Interproximal gingiva 53. The collar-like band of stratified squamous epithelium 10-20 cells thick coronally and 2-3 cells thick apically, and .25 to 1.35 mm long is the: 1.