Download Vol. 3, No. 5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Do Worm Lizards Occur in Nebraska? Louis A
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Herpetology Papers in the Biological Sciences 1993 Do Worm Lizards Occur in Nebraska? Louis A. Somma Florida State Collection of Arthropods, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/biosciherpetology Part of the Biodiversity Commons, and the Population Biology Commons Somma, Louis A., "Do Worm Lizards Occur in Nebraska?" (1993). Papers in Herpetology. 11. http://digitalcommons.unl.edu/biosciherpetology/11 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Herpetology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. @ o /' number , ,... :S:' .' ,. '. 1'1'13 Do Mono Li ••rel,. Occur ill 1!I! ..br .... l< .. ? by Louis A. Somma Department of- Zoology University of Florida Gainesville, FL 32611 Amphisbaenids, or worm lizards, are a small enigmatic suborder of reptiles (containing 4 families; ca. 140 species) within the order Squamata, which include~ the more speciose lizards and snakes (Gans 1986). The name amphisbaenia is derived from the mythical Amphisbaena (Topsell 1608; Aldrovandi 1640), a two-headed beast (one head at each end), whose fantastical description may have been based, in part, upon actual observations of living worm lizards (Druce 1910). While most are limbless and worm-like in appearance, members of the family Bipedidae (containing the single genus Sipes) have two forelimbs located close to the head. This trait, and the lack of well-developed eyes, makes them look like two-legged worms. -

Origin of Tropical American Burrowing Reptiles by Transatlantic Rafting
Biol. Lett. in conjunction with head movements to widen their doi:10.1098/rsbl.2007.0531 burrows (Gans 1978). Published online Amphisbaenians (approx. 165 species) provide an Phylogeny ideal subject for biogeographic analysis because they are limbless (small front limbs are present in three species) and fossorial, presumably limiting dispersal, Origin of tropical American yet they are widely distributed on both sides of the Atlantic Ocean (Kearney 2003). Three of the five burrowing reptiles by extant families have restricted geographical ranges and contain only a single genus: the Rhineuridae (genus transatlantic rafting Rhineura, one species, Florida); the Bipedidae (genus Nicolas Vidal1,2,*, Anna Azvolinsky2, Bipes, three species, Baja California and mainland Corinne Cruaud3 and S. Blair Hedges2 Mexico); and the Blanidae (genus Blanus, four species, Mediterranean region; Kearney & Stuart 2004). 1De´partement Syste´matique et Evolution, UMR 7138, Syste´matique, Evolution, Adaptation, Case Postale 26, Muse´um National d’Histoire Species in the Trogonophidae (four genera and six Naturelle, 57 rue Cuvier, 75231 Paris Cedex 05, France species) are sand specialists found in the Middle East, 2Department of Biology, 208 Mueller Laboratory, Pennsylvania State North Africa and the island of Socotra, while the University, University Park, PA 16802-5301, USA largest and most diverse family, the Amphisbaenidae 3Centre national de se´quenc¸age, Genoscope, 2 rue Gaston-Cre´mieux, CP5706, 91057 Evry Cedex, France (approx. 150 species), is found on both sides of the *Author and address for correspondence: De´partment Syste´matique et Atlantic, in sub-Saharan Africa, South America and Evolution, UMR 7138, Syste´matique, Evolution, Adoptation, Case the Caribbean (Kearney & Stuart 2004). -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -
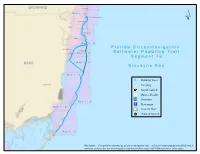
Segment 16 Map Book
Hollywood BROWARD Hallandale M aa p 44 -- B North Miami Beach North Miami Hialeah Miami Beach Miami M aa p 44 -- B South Miami F ll o r ii d a C ii r c u m n a v ii g a tt ii o n Key Biscayne Coral Gables M aa p 33 -- B S a ll tt w a tt e r P a d d ll ii n g T r a ii ll S e g m e n tt 1 6 DADE M aa p 33 -- A B ii s c a y n e B a y M aa p 22 -- B Drinking Water Homestead Camping Kayak Launch Shower Facility Restroom M aa p 22 -- A Restaurant M aa p 11 -- B Grocery Store Point of Interest M aa p 11 -- A Disclaimer: This guide is intended as an aid to navigation only. A Gobal Positioning System (GPS) unit is required, and persons are encouraged to supplement these maps with NOAA charts or other maps. Segment 16: Biscayne Bay Little Pumpkin Creek Map 1 B Pumpkin Key Card Point Little Angelfish Creek C A Snapper Point R Card Sound D 12 S O 6 U 3 N 6 6 18 D R Dispatch Creek D 12 Biscayne Bay Aquatic Preserve 3 ´ Ocean Reef Harbor 12 Wednesday Point 12 Card Point Cut 12 Card Bank 12 5 18 0 9 6 3 R C New Mahogany Hammock State Botanical Site 12 6 Cormorant Point Crocodile Lake CR- 905A 12 6 Key Largo Hammock Botanical State Park Mosquito Creek Crocodile Lake National Wildlife Refuge Dynamite Docks 3 6 18 6 North Key Largo 12 30 Steamboat Creek John Pennekamp Coral Reef State Park Carysfort Yacht Harbor 18 12 D R D 3 N U O S 12 D R A 12 C 18 Basin Hills Elizabeth, Point 3 12 12 12 0 0.5 1 2 Miles 3 6 12 12 3 12 6 12 Segment 16: Biscayne Bay 3 6 Map 1 A 12 12 3 6 ´ Thursday Point Largo Point 6 Mary, Point 12 D R 6 D N U 3 O S D R S A R C John Pennekamp Coral Reef State Park 5 18 3 12 B Garden Cove Campsite Snake Point Garden Cove Upper Sound Point 6 Sexton Cove 18 Rattlesnake Key Stellrecht Point Key Largo 3 Sound Point T A Y L 12 O 3 R 18 D Whitmore Bight Y R W H S A 18 E S Anglers Park R 18 E V O Willie, Point Largo Sound N: 25.1248 | W: -80.4042 op t[ D A I* R A John Pennekamp State Park A M 12 B N: 25.1730 | W: -80.3654 t[ O L 0 Radabo0b. -

Reptiles in Arkansas
Terrestrial Reptile Report Carphophis amoenus Co mmon Wor msnake Class: Reptilia Order: Serpentes Family: Colubridae Priority Score: 19 out of 100 Population Trend: Unknown Global Rank: G5 — Secure State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Arkansas Valley Ouachita Mountains South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plain Carphophis amoenus Common Wormsnake 1079 Terrestrial Reptile Report Habitat Map Habitats Weight Crowley's Ridge Loess Slope Forest Obligate Lower Mississippi Flatwoods Woodland and Forest Suitable Problems Faced KNOWN PROBLEM: Habitat loss due to conversion Threat: Habitat destruction or to agriculture. conversion Source: Agricultural practices KNOWN PROBLEM: Habitat loss due to forestry Threat: Habitat destruction or practices. conversion Source: Forestry activities Data Gaps/Research Needs Genetic analyses comparing Arkansas populations with populations east of the Mississippi River and the Western worm snake. Conservation Actions Importance Category More data are needed to determine conservation actions. Monitoring Strategies More information is needed to develop a monitoring strategy. Carphophis amoenus Common Wormsnake 1080 Terrestrial Reptile Report Comments Trauth and others (2004) summarized the literature and biology of this snake. In April 2005, two new geographic distribution records were collected in Loess Slope Forest habitat within St. Francis National Forest, south of the Mariana -

TRAPPING SUCCESS and POPULATION ANALYSIS of Siren Lacertina and Amphiuma Means
TRAPPING SUCCESS AND POPULATION ANALYSIS OF Siren lacertina AND Amphiuma means By KRISTINA SORENSEN A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2003 ACKNOWLEDGMENTS I thank my committee members Lora Smith, Franklin Percival, and Dick Franz for all their support and advice. The Department of Interior's Student Career Experience Program and the U.S. Geological Survey's Amphibian Research and Monitoring Initiative provided funding for this project. I thank those involved with these programs who have helped me over the last three years: David Trauger, Ken Dodd, Jamie Barichivich, Jennifer Staiger, Kevin Smith, and Steve Johnson. Numerous people helped with field work: Audrey Owens, Maya Zacharow, Chris Gregory, Matt Chopp, Amanda Rice, Paul Loud, Travis Tuten, Steve Johnson, and Jennifer Staiger, Lora Smith, and the UF Wildlife Field Techniques Courses of2001-2002. Paul Moler and John Jensen provided advice and shared their wealth of herpetological knowledge. I thank the staff of Okefenokee National Wildlife Refuge and Steve Coates, manager of the Ordway Preserve, for their assistance on numerous occasions and for permission to conduct research on their property. Marinela Capanu of the IFAS Statistical Consulting Unit assisted with statistical analysis. Julien Martin, Bob Dorazio, Rob Bennets, and Cathy Langtimm provided advice on population analysis. I also thank the administrative staff of the Florida Caribbean Science Center and the Florida Cooperative Fish and Wildlife Research Unit. I am much indebted to all of these people, without whom this thesis would not have been possible. -

Florida Coral Reefs: Islandia
FOREWORD· In their present relatively undeveloped state, the upper Florida Keys and the adjoining waters and submerged. lands of Biscayne ~ay and the Atlantic Ocean are an enviromr~.ental element highly important to Florida ancl a valuable recreation resource for the nation. Fully aware that intensive private development would greatly alter . these values, the Secretary of the Interior directed the National Park Service and the Bureau of Outdoor Recreation, assisted by the Fish and Wildlife Service, to conduct studies of the area. This interim professional report is the r~sult of these studies. It is being distributed now to solicit the comments and suggestions of interested parties. The additional information obtained in this manner will be utilized by the National Park Service and the Bureau of Outdoor Recre ation to complete the study and formulate recommendations to the Secretary· of the Interior. It is requested that comments and suggestions on this interim profes sional report be sent to the Regional Director, Southeast Region, Na tional Park Service, P. 0. Box 10008, Richmond, Virginia 23240. Material should be submitted in time to reach the Regional Director on or before August 15, 1965. ~~ Edward C. Crafts Director Director Bureau of Outdoor Recreation National Park Service Page No. FOREWORD THE STUDY 2 THE CORAL REEFS The Resource 3 The Climate 5 The Ecology 6 MAN AND THE CORAL REEFS 10 THE SITUATION Development Possibilities · 12 Significance for Preservation 12 THE OPPORTUNITIES 18 Alternative Plans 14' Plan 1 16 Plan 2 20 Plan 3 24 THE STUDY In response to interest expressed by the weekending, and vacationing are engaged Dade Co1:-1nty Board of Commissioners and in extensively. -

Evolution of the Iguanine Lizards (Sauria, Iguanidae) As Determined by Osteological and Myological Characters
Brigham Young University BYU ScholarsArchive Theses and Dissertations 1970-08-01 Evolution of the iguanine lizards (Sauria, Iguanidae) as determined by osteological and myological characters David F. Avery Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Life Sciences Commons BYU ScholarsArchive Citation Avery, David F., "Evolution of the iguanine lizards (Sauria, Iguanidae) as determined by osteological and myological characters" (1970). Theses and Dissertations. 7618. https://scholarsarchive.byu.edu/etd/7618 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. EVOLUTIONOF THE IGUA.NINELI'ZiUIDS (SAUR:U1., IGUANIDAE) .s.S DETEH.MTNEDBY OSTEOLOGICJJJAND MYOLOGIC.ALCHARA.C'l'Efi..S A Dissertation Presented to the Department of Zoology Brigham Yeung Uni ver·si ty Jn Pa.rtial Fillf.LLlment of the Eequ:Lr-ements fer the Dz~gree Doctor of Philosophy by David F. Avery August 197U This dissertation, by David F. Avery, is accepted in its present form by the Department of Zoology of Brigham Young University as satisfying the dissertation requirement for the degree of Doctor of Philosophy. 30 l'/_70 ()k ate Typed by Kathleen R. Steed A CKNOWLEDGEHENTS I wish to extend my deepest gratitude to the members of m:r advisory committee, Dr. Wilmer W. Tanner> Dr. Harold J. Bissell, I)r. Glen Moore, and Dr. Joseph R. Murphy, for the, advice and guidance they gave during the course cf this study. -

Index to Scientific Names of Amphibians and Reptiles for Volume 33 (1998)
Bull. Chicago Herp. Soc. 33(12):271-273, 1998 Index to Scientific Names of Amphibians and Reptiles for Volume 33 (1998) January 1-24 April 73-96 July 141-160 October 205-224 February 25-48 May 97-112 August 161-180 November 225-252 March 49-72 June 113-140 September 181-204 December 253-276 Abronia 20 slevini 19 fowleri 191-193 neotesselatus 75, 76, 79-82 Acanthodactylus steindachneri 19 Caiman neotesselatus × sexlineatus 82 bedriagai 182, 184 Apalone 91 crocodilus crocodilus 109, 177 septemvittatus 77 boskianus 185 mutica 148 yacare 177 sexlineatus 6-8, 75-81, 83, 144, dumerili exiguus 182, 185 spinifera 144-146, 239, 241 Callisaurus draconoides 52 146 erythrurus lineomaculatus 185 spinifera 40 Calloselasma rhodostoma 220 tesselatus 12, 75-84 Acris crepitans 143, 144, 152, 239, Arthrosaura synaptolepis 41 Cerastes tesselatus × sexlineatus 79-80 240 Atractus 250 cerastes 187 tigris 77 Acrochordus arafurae 68 Batrachoseps 220 vipera 187 marmoratus 78 Adelphicos 250 nigriventris 109 Cerberus rynchops 109 vanzoi 268 Afroedura 93 wrighti 109 Chalcides Coleonyx 20 Agama impalearis 183 Bipes 1-5 mionecton 185 Colostethus 92 Agalychnis callidryas 212-214 biporus 1, 2 ocellatus atopoglossus 92 Agkistrodon 32 canaliculatus 2 ocellatus 185 lacrimosus 92 contortrix 259, 260 Bitis caudalis 248 tiligugu 185 tamacuarensis 41 mokasen 189, 190 Blanus 2 Chameleo Coluber piscivorus 250, 258, 259 cinereus 2, 3 africanus 184 algirus algirus 182, 186 leucostoma 100 tingitanus 183 chameleon chameleon 184 constrictor 9-10, 12, 148, 239, 241 Aipysurus laevis -

Herpetofauna Occupancy and Community Composition Along a Tidal Swamp Salinity Gradient Sidney Thomas Godfrey Clemson University, [email protected]
Clemson University TigerPrints All Theses Theses 5-2018 Herpetofauna Occupancy and Community Composition Along a Tidal Swamp Salinity Gradient Sidney Thomas Godfrey Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_theses Recommended Citation Godfrey, Sidney Thomas, "Herpetofauna Occupancy and Community Composition Along a Tidal Swamp Salinity Gradient" (2018). All Theses. 2840. https://tigerprints.clemson.edu/all_theses/2840 This Thesis is brought to you for free and open access by the Theses at TigerPrints. It has been accepted for inclusion in All Theses by an authorized administrator of TigerPrints. For more information, please contact [email protected]. HERPETOFAUNA OCCUPANCY AND COMMUNITY COMPOSITION ALONG A TIDAL SWAMP SALINITY GRADIENT A Thesis Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Master of Science Wildlife and Fisheries Biology by Sidney Thomas Godfrey May 2018 Accepted by: Dr. Robert Baldwin, Committee Chair Dr. Jamie Duberstein Dr. William Conner Dr. Hardin Waddle Dr. William Bridges i ABSTRACT Tidal swamps provide habitats for a variety of reptiles and amphibians (herpetofauna), but their community compositions in most tidal swamps are currently unknown. These swamps currently face a number of threats, such as saltwater intrusion, yet the impacts to herpetofaunal communities have not been assessed. Saltwater intrusions into the upper reaches of coastal rivers contribute to their salinity gradients, which can influence associated plant and animal communities. Our study assessed the reptile and amphibian diversity along a salinity gradient in the upper estuary of the Savannah River to further predictive capabilities regarding herpetofauna. -

Report T -622 the Status of Florida Tree Snails (Liguus Fasciatus)
DANIEL SCHEIDT -... --_ .-- -..... -~- -~ . Report T-622 The Status of Florida Tree Snails (Liguus fasciatus), Introduced to Everglades National Park Everglades National Park, South Florida Research Center, P.O. Box 279, Homestead, Florida 33030 The Status of Florida Tree Snails (Liguus fasciatus), Introduced to Everglades National Park Report T-622 Archie L. Jones, Erwin C. Winte and Oron L. Bass, Jr. National Park Service South Florida Research Center Everglades National Park Homestead, Florida 33030 April 1981 · 0010 Jones, Archie L., Erwin C. Winte and Oron L. Bass, Jr. 1981. The Status of Florida Tree Snails (Liguus fasciatus), Introduced to Everglades National Park. South Florida Research Center Report T -622. 31 pp. TABLE OF CONTENTS INTRODUC TION . • . • • • . • • • • • • • • • • • 1 GENERAL HISTORY, TAXONOMY AND DISTRIBUTION •• 1 History and Taxonomy. 1 Distr ibution • • • 2 PROJECT HISTORY. • 2 METHODS .••••• 3 Introduction Area. • 3 Mapping. • • • • . 3 STATUS OF COLOR FORMS 3 INTRODUCTION SITES • • 10 Figure 1. Distribution and geographical regions of Florida tree snails 27 Figure 2. Area of introduced Florida tree snails in Everglades National Park. 28 Table 1. The 58 color forms of the Florida tree snail, Liguus fasciatus. • 29 LITERATURE CITED • • • • • 30 1 INTRODUCTION The Florida tree snail, Liguus fasciatus, in the family Bulimulidae, is unique among land snails in North Am erica because of its bright colors and variable patterns. This species is tropical in origin being derived from West Indian forms. It is restricted to the tropical hardwood hammocks scattered throughout south Florida including the Florida Keys. There are 58 named color forms. Of these, 10 are extinct in their native habitat and 4 may soon become extinct. -

Okefenokee National Wildlife Refuge Amphibians, Fish, Mammals and Reptiles List
U.S. Fish & Wildlife Service Okefenokee National Wildlife Refuge Amphibians, Fish, Mammals and Reptiles List The Okefenokee swamp is covered with Mammals ___Seminole Bat cypress, blackgum, and bay forests (Lasiurus seminolus). A common bat of scattered throughout a flooded prairie ___Virginia Opossum the Okefenokee which is found hanging in made of grasses, sedges, and various (Didelphis virginiana pigna). Common on Spanish Moss during the day. the swamp edge and the islands within aquatic plants. The peripheral upland and ___Hoary Bat the almost 70 islands within the swamp the Swamp. A night prowler. “Pogo” is often seen by campers. (Lasiurus cinereus cinereus). This are forested with pine interspersed with yellowish-brown bat flies high in the air hardwood hammocks. Lakes of varying ___Southern Short-Tailed Shrew late at night and will hang in trees when sizes and depths, and floating sections (Barina carolinensis). A specimen was resting. It is the largest bat in the East of the peat bed, are also part of the found on Floyds Island June 12, 1921. It and eats mostly moths. Okefenokee terrain. kills its prey with poisonous saliva. ___Northern Yellow Bat People have left their mark on the swamp. ___Least Shrew (Lasiurus intermedius floridanus). A 12-mile long canal was dug into the (Cryptotus parva parva). Rarely seen but Apparently a rare species in the area. It eastern prairies in the 1890’s in a failed probably fairly common. Specimens have likes to feed in groups. attempt to drain the swamp. During the been found on several of the islands, on early 1900’s large amounts of timber were the swamp edge, and in the pine woods ___Evening Bat removed, so that very few areas of virgin around the swamp.