A Review on Phytochemicals of the Genus Maytenus and Their Bioactive Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter14.Pdf
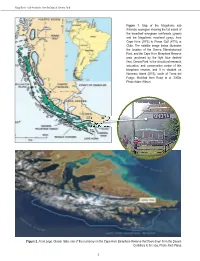
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

Marketplace Plants Used in Ceremonial Cleansing Among Andean Qechuans of Ecuador
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Marshall University Marshall University Marshall Digital Scholar Theses, Dissertations and Capstones 2007 Marketplace plants used in ceremonial cleansing among Andean Qechuans of Ecuador Sushma Shrestha Follow this and additional works at: https://mds.marshall.edu/etd Part of the Folklore Commons, Indigenous Studies Commons, and the Social and Cultural Anthropology Commons Recommended Citation Shrestha, Sushma, "Marketplace plants used in ceremonial cleansing among Andean Qechuans of Ecuador" (2007). Theses, Dissertations and Capstones. 1275. https://mds.marshall.edu/etd/1275 This Thesis is brought to you for free and open access by Marshall Digital Scholar. It has been accepted for inclusion in Theses, Dissertations and Capstones by an authorized administrator of Marshall Digital Scholar. For more information, please contact [email protected], [email protected]. Marketplace plants used in ceremonial cleansing among Andean Qechuans of Ecuador Thesis submitted to The Graduate School of Marshall University In partial fulfilment of the Requirements for the degree of Master of Science in Biological Sciences by Sushma Shrestha Dr. Dan K. Evans, Ph.D., Chairperson Dr. Charles Somerville, Ph.D. Dr. Tom Pauley, Ph.D. Marshall University 2007 ii TO MY FAMILY and INDIGINOUS PEOPLE AROUND THE WORLD iii ACKNOWLEDGEMENT I am grateful to Dr. Evans for further igniting my interest in plant and indigenous people. I appreciate all your help in Ecuador and here during the research and beyond with both academic and financial support for the work. You are a wonderful professor, advisor and a travel companion. -

Bosque Pehuén Park's Flora: a Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile Author(S): Daniela Mellado-Mansilla, Iván A
Bosque Pehuén Park's Flora: A Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile Author(s): Daniela Mellado-Mansilla, Iván A. Díaz, Javier Godoy-Güinao, Gabriel Ortega-Solís and Ricardo Moreno-Gonzalez Source: Natural Areas Journal, 38(4):298-311. Published By: Natural Areas Association https://doi.org/10.3375/043.038.0410 URL: http://www.bioone.org/doi/full/10.3375/043.038.0410 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. R E S E A R C H A R T I C L E ABSTRACT: In Chile, most protected areas are located in the southern Andes, in mountainous land- scapes at mid or high altitudes. Despite the increasing proportion of protected areas, few have detailed inventories of their biodiversity. This information is essential to define threats and develop long-term • integrated conservation programs to face the effects of global change. -

(Mart. Ex Reiss.) Biral Leaves and Its Adulterants Sold As Medicinal Tea in Brazil: a Contribution to Quality Control
BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Articulo Original / Original Article Pharmacobotanical characterization of Monteverdia ilicifolia (Mart. ex Reiss.) Biral leaves and its adulterants sold as medicinal tea in Brazil: a contribution to quality control [Caracterización farmacobotánica de hojas de Monteverdia ilicifolia (Mart. ex Reiss.) Biral y sus adulterantes vendidos como té medicinal en Brasil: una contribución al control de calidad] Fernanda Moreira do Amaral1,2, Sarah de Sá Rego Monteiro1,3, Thiago Fernandes1,4, Dulcinéia Furtado Teixeira5,6, Leonardo Lucchetti6, Silvana do Couto Jacob5, Selma Ribeiro de Paiva1 & Ana Joffily1 1Instituto de Biologia, Universidade Federal Fluminense, Niterói, Brasil 2Programa de Pós-Graduação em Ciências Biológicas, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil 3Curso de Graduação em Ciências Biológicas, Faculdades Integradas Maria Thereza, Niterói, Brasil 4Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rio de Janeiro, Brasil 5Programa de Pós-Graduação em Vigilância Sanitária (INCQS), Fundação Oswaldo Cruz, Rio de Janeiro, Brasil 6Fundação Oswaldo Cruz, Rio de Janeiro, Brasil Abstract: Leaves of Monteverdia ilicifolia (“espinheira-santa”) are considered a medicinal tea by the Brazilian Sanitary Surveillance Agency (Anvisa), by their anti-dyspeptic, anti-acid and protective of the Reviewed by: gastric mucosa properties. Their spiny margins are similar to those of other botanical species, which may Graciela Ponessa lead to misidentifications. The aim of this work was to evaluate the authenticity of 32 samples of herbal Fundación Miguel Lillo drugs commercialized as “espinheira-santa” in the formal trade in Brazil, by macro and microscopic Argentina morphological studies of the leaves. -

FACULTAD DE FARMACIA Y BIOQUÍMICA Bach. Winnie Kenny
"AÑO DE LA DIVERSIFICACIÓN PRODUCTIVA Y DEL FORTALECIMIENTO DE LA EDUCACIÓN" UNIVERSIDAD NACIONAL "SAN LUIS GONZAGA" DE ICA FACULTAD DE FARMACIA Y BIOQUÍMICA "EVALUACIÓN DE LA ACTIVIDAD ANTIOXIDANTE Y ANTIBACTERIANA DEL EXTRACTO ETANÓLICO DE lAS HOJAS DE Maytenus octogona {L' Héritier) DC." Tesis para optar el Título Profesional de: QUÍMICO FARMACÉUTICO Presentado por: Bach. Winnie Kenny, Caile Condor Bach. Vanessa Patricia, Gutierrez Alvarado Asesores: Dra. HAYDEÉ CHAVEZ ORELLANA Dra. CARMEN HUAYANGA GUTIÉRREZ Dr. FELIPE SURCO LAOS ICA- PERÚ 2015 DEDICATORIA A Dios: Por damos s1empre las fuerzas para continuar en lo adverso, por guiamos en el sende,ro de lo sensato y damos sabiduría en las situaciones dificiles. A mis padres: Oiga y Emiliano Por su amor, trabajo y saerifieio en todos estos atios. Por sus eonsejos, su apoyo constante, a quienes debo mi formación personal y profesional con mi gratitud, cariño, respeto y admiración. A mis hermanas: Mirlam y Maggie Por la confianza depositada en mí. Por la complicidad de muchos años. Y porque siempre serán mi ejemplo a seguir. A mis sobrinos: Kenyiro y Kenny Porque trajeron muchas alegrías con sus llegadas, este logro es por ustedes. A mi abuela: Epifania (QÉPO), Por su cariño, sus cuidados y porque desde el lugar en que esté siempre está cuidándome. A mis familiares y amigos: Por todo el apoyo que me brindaron desde siempre y por su compañía en momentos de dificultades. A Vanessa Gutierrez Alvarado: Por su amistad ineondieicmal, en los buenos y malos momentos. Por eompartir conmigo sus conocimientos, alegrías y tristezas desde el comienzo de esta carrera. -

An Overview on Ethanomedicinal Plant Gymnosporia Montana of Celestraceae Family
International Journal of Pharmacognosy and Chinese Medicine ISSN: 2576-4772 An Overview on Ethanomedicinal Plant Gymnosporia Montana of Celestraceae Family Bhavita D1*, Lakshmi B2 and Maitreyi Z3 Research Article Volume 1 Issue 1 1Department of Pharmacognosy, KB Institute of Pharmaceutical Education and Received Date: March 08, 2017 Research, India Published Date: March 24, 2017 2Department of Pharmacognosy, KB Institute of Pharmaceutical Education and Research, India 3Department of Pharmacognosy, KB Institute of Pharmaceutical Education and Research, India *Corresponding author: Dhru Bhavita, Department of Pharmacognosy, KB Institute of Pharmaceutical Education and Research, Gujarat, India, E-mail: [email protected] Abstract Ethanomedicinal plant like Gymnosporia montana belonging to the family Celeastraceae commonly known as Vikalo in Gujarat. Ethan medicinally fresh leaves of Vikalo are chewed in tribal regions of Gujarat to cure jaundice. The present review includes ethnomedicinal, pharmacognostical, phytochemical and pharmacological activity of Gymnosporia montana. Keywords: Gymnosporia Montana; Vikalo; Celeastraceae; Ethanomedicinal Introduction have also been described. Diterpene triepoxides with potent antileukemic and immunosuppressive activities The use of medicinal plants for the treatment of and triterpenoid quinonemethides named as various diseases is as old as human civilization and has “Celastroloids” with antibiotic and cytostatic activities obtained a worldwide significance in the primary have been isolated from species of the Celesteraceae healthcare system. In spite of their structural family [1]. complexicity and many unknown chemical constituents, they have been frequently prescribed because of their use Family Celastraceae contain about 90-100 genera and and efficacy, contributing to the disclosure of their 1,300 species of vines, shrubs, usually of 1-9 m height therapeutic properties. -

Chuchuhuasi Tech Report
Technical Data Report for CHUCHUHUASI Maytenus krukovii All rights reserved. No part of this document may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage or retrieval system, without written permission from Sage Press, Inc. This document is not intended to provide medical advice and is sold with the understanding that the publisher and the author are not liable for the misconception or misuse of information provided. The author and Sage Press, Inc. shall have neither liability nor responsibility to any person or entity with respect to any loss, damage, or injury caused or alleged to be caused directly or indirectly by the infor- mation contained in this document or the use of any plants mentioned. Readers should not use any of the products discussed in this document without the advice of a medical professional. © Copyright 2003 Sage Press, Inc., P.O. Box 80064, Austin, TX 78708-0064. All rights reserved. CHUCHUHUASI Family: Celastraceae Genus: Maytenus Species: krukovii Synonyms: Maytenus ebenifolia, M. laevis, M. macrocarpa, M. multiflora, M. terapotensis, Celastrus macrocarpus, Haenkea macrocarpa, H. multiflora Common Names: Chuchuhuasi, chucchu huashu, chuchuasi, chuchasha, chuchuhuasha Parts Used: Bark, root, leaves HERBAL PROPERTIES AND ACTIONS Main Actions Other Actions Standard Dosage ! reduces inflammation ! kills cancer cells Bark ! relieves pain ! prevents tumors Decoction: 1 cup 2-3 times daily ! relaxes muscles ! stimulates digestion Tincture: 3-5 ml 2-3 times daily ! enhances immunity ! increases libido ! supports adrenals Chuchuhuasi is an enormous canopy tree of the Amazon rainforest that grows to 30 m high. -

TAXON:Fuchsia Magellanica Lam. SCORE:18.0 RATING:High Risk
TAXON: Fuchsia magellanica Lam. SCORE: 18.0 RATING: High Risk Taxon: Fuchsia magellanica Lam. Family: Onagraceae Common Name(s): earring flower Synonym(s): Fuchsia gracilis Lindl. hardy fuchsia Fuchsia macrostemma Ruiz & Pav. kulapepeiao lady's eardrops Assessor: Chuck Chimera Status: Assessor Approved End Date: 9 Jul 2021 WRA Score: 18.0 Designation: H(HPWRA) Rating: High Risk Keywords: Smothering Shrub, Environmental Weed, Self-Compatible, Spreads Vegetatively, Bird- Dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 y Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) y 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) y 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 n 405 Toxic to animals y=1, n=0 n 406 Host for recognized pests and pathogens 407 Causes allergies or is otherwise toxic to humans y=1, n=0 n Creation Date: 9 Jul 2021 (Fuchsia magellanica Lam. -

9. a 10 Year Trial with South American Trees and Shrubs with Special
9. A 10 year trial with SouthAmerican trees and shrubswith specialregard to the Ir,lothofaglzsspp. I0 6ra royndir vid suduramerikonskumtroum og runnum vid serligumatliti at Nothofagw-slogum SarenOdum Abstract The potential of the ligneous flora of cool temperate South America in arboriculture in the Faroe Isles is elucidated through experimental planting of a broad variety of speciescollected on expeditions to Patagonia and Tierra del Fuego 1975 andl9T9.Particular good results have been obtained with the southernmost origins of Nothofagus antarctica, N. betuloides, and N. pumilio, of which a total of 6.500 plants were directly transplanted from Tierra del Fuego to the Faroe Isles in 1979. Soren Odum, Royal Vet.& Agric. IJniv., Arboretum, DK-2970 Horsholm, Denmark. Introduction As a student of botany at the University of CopenhagenI got the opportunity to get a job for the summer 1960as a member of the team mapping the flora of the Faroe Isles (Kjeld Hansen 1966). State geologist of the Faroe Isles and the Danish Geological Survey, J6annesRasmussen, provided working facilities for the team at the museum, and also my co-student,J6hannes J6hansen participated in the field. This stay and work founded my still growing interest in the Faroese nature and culture, and the initial connections between the Arboretum in Horsholm and Tbrshavn developed from this early contact with J6annesRasmussen and J6hannes J6hansen. On our way back to Copenhagen in 1960 onboard "Tjaldur", we called on Lerwick, Shetland, where I saw Hebe and Olearia in some gardens. This made it obvious to me, that if the Faroe Isles for historical reasonshad been more or less British rather than Nordic, the gardensof T6rshavn would, no doubt, have been speckledwith genera from the southern Hemisphere and with other speciesand cultivars nowadays common in Scottish nurseries and gardens. -

Palaeoenvironmental Changes in Southern Patagonia During the Late-Glacial and the Holocene: Implications
Palaeoenvironmental changes in southern Patagonia during the Late-glacial and the Holocene: implications for forest establishment and climate reconstructions. Claudia A. Mansilla Submitted for the degree of Doctor of Philosophy Biological and Environmental Sciences School of Natural Sciences University of Stirling 2015 STATEMENT OF ORIGINALITY I hereby confirm that the research contained in this thesis is original, has been completed by the author and all work contained herein has not been submitted for any other degree. All published material has been duly acknowledged and cited. ………………………………………………………………….. Claudia A. Mansilla Date: ii ABSTRACT Three continuous terrestrial high-resolution palaeoenvironmental records for the Late-glacial and the Holocene have been reconstructed for different ecosystems in Fuego-Patagonia on a longitudinal transect at latitude 53°S. The records describe the nature and extent of environmental and climatic changes inferred from palynological evidence supported by lithostratigraphy, tephrochronology and radiocarbon dating. The environmental changes recorded at the three sites displays a significant degree of synchrony in response to similar large-scale climatic changes. Clear stratigraphical evidence alongside the pollen record indicates a shift to warmer interstadial conditions between c. 14,800 Cal yr BP and 14,400 Cal yrs BP. During the period coeval with ACR the vegetation was dominated by cold resistant dry land herbs such as Poaceae, Asteraceae (Suf. Asteroideae) and Acaena, by c. 13,200 Cal yr BP the vegetation changed from the dominance of cold resistant dry land herbs towards more mesic conditions and the expansion of steppe dominated by Poaceae with patches of Nothofagus forest. The establishment of the forest and an eastward shift of the forest-steppe ecotone by c. -

Aspectos Reproductivos, Arquitectura Y Fenomorfología De Maytenus
GAYANA BOTANICA Gayana Bot. (2020) vol. 77, No. 2, 152-167 DOI: ORIGINAL ARTICLE Aspectos reproductivos, arquitectura y fenomorfología de Maytenus boaria Molina (Celastraceae) en Chile central Reproductives aspects, architecture and phenomorphology of Maytenus boaria Molina (Celastraceae) in central Chile Magdalena Godoy1, Luz María de la Fuente3, Miguel Gómez2 & Rosanna Ginocchio1,3* 1Departamento de Ecosistemas y Medio Ambiente, Facultad de Agronomía e Ingeniería Forestal, Pontificia Universidad Católica de Chile, Santiago, Chile. 2Departamento de Ciencias Vegetales, Facultad de Agronomía e Ingeniería Forestal, Pontificia Universidad Católica de Chile, Santiago, Chile. 3Center of Applied Ecology and Sustainability (CAPES), Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile. *[email protected] RESUMEN Maytenus boaria Molina (Celastraceae) es un árbol nativo siempreverde de amplia distribución en Chile, con alto potencial económico por los diversos usos tradicionales descritos. A pesar de esto, existe escasa y confusa información biológica de la especie. El objetivo de este estudio fue ampliar el conocimiento sobre aspectos reproductivos y el sistema sexual de M. boaria, además de la arquitectura de la copa y su fenomorfología. El estudio se realizó entre agosto y diciembre de 2016, con individuos adultos (n=17) seleccionados en sectores urbanos de la Región Metropolitana. Se realizó un ensayo de polinización y se identificó el sexo de las flores de cada árbol seleccionado. Además, se realizó un diagrama fenomorfológico de la especie a través de la interpretación modular de la arquitectura y la identificación de sus fenofases. Los resultados indican que M. boaria es dioica y andromonoica; presenta una baja autogamia autónoma y es una especie no apomíctica que necesita de vectores de polinización para la producción de semillas. -

Reinstatement of Gymnosporia (Celastraceae): Implications for the Flora Malesiana Region
155 Reinstatement of Gymnosporia (Celastraceae): implications for the Flora Malesiana region Marie Jordaan and A.E. van Wyk Abstract Jordaan, Marie1 and van Wyk, A.E.2 (1National Botanical Institute, Private Bag X101, Pretoria, Republic of South Africa 0001; e-mail: [email protected]; 2Department of Botany, University of Pretoria, Pretoria, Republic of South Africa 0002; e-mail: [email protected]) 2003. Reinstatement of Gymnosporia (Celastraceae): implications for the Flora Malesiana region. Telopea 10(1): 155–167. The reinstatement of the genus Gymnosporia, comprising all the spiny members previously included in the genus Maytenus, has implications for the Flora Malesiana region. An account is given of six species and two varieties in the region (Gymnosporia curtisii, G. diversifolia, G. inermis, G. littoralis, G. nitida, G. spinosa var. spinosa and var. parva). The new combination G. littoralis (Backer) Jordaan is made (based on Gymnosporia montana var. littoralis) and a neotype and lectotype designated for two other taxa. G. emarginata from India and Sri Lanka is also included since this name was previously misapplied to plants in the Flora Malesiana region. Maytenus rapakir is mentioned as probably belonging to Gymnosporia. Maytenus cupularis, which has a racemose inflorescence, is related to the Australian species of Maytenus s. lat. and is retained in Maytenus. Introduction The Celastraceae account for the Flora Malesiana was published by Ding Hou in 1962 and 1964. His genus concepts coincide with those of Exell (1952, 1953), Blakelock (1956), Marais (1960) and Robson (1965, 1966 & 1994) in that he considers Gymnosporia and Maytenus to be congeneric.