Electron Diffraction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classical and Modern Diffraction Theory
Downloaded from http://pubs.geoscienceworld.org/books/book/chapter-pdf/3701993/frontmatter.pdf by guest on 29 September 2021 Classical and Modern Diffraction Theory Edited by Kamill Klem-Musatov Henning C. Hoeber Tijmen Jan Moser Michael A. Pelissier SEG Geophysics Reprint Series No. 29 Sergey Fomel, managing editor Evgeny Landa, volume editor Downloaded from http://pubs.geoscienceworld.org/books/book/chapter-pdf/3701993/frontmatter.pdf by guest on 29 September 2021 Society of Exploration Geophysicists 8801 S. Yale, Ste. 500 Tulsa, OK 74137-3575 U.S.A. # 2016 by Society of Exploration Geophysicists All rights reserved. This book or parts hereof may not be reproduced in any form without permission in writing from the publisher. Published 2016 Printed in the United States of America ISBN 978-1-931830-00-6 (Series) ISBN 978-1-56080-322-5 (Volume) Library of Congress Control Number: 2015951229 Downloaded from http://pubs.geoscienceworld.org/books/book/chapter-pdf/3701993/frontmatter.pdf by guest on 29 September 2021 Dedication We dedicate this volume to the memory Dr. Kamill Klem-Musatov. In reading this volume, you will find that the history of diffraction We worked with Kamill over a period of several years to compile theory was filled with many controversies and feuds as new theories this volume. This volume was virtually ready for publication when came to displace or revise previous ones. Kamill Klem-Musatov’s Kamill passed away. He is greatly missed. new theory also met opposition; he paid a great personal price in Kamill’s role in Classical and Modern Diffraction Theory goes putting forth his theory for the seismic diffraction forward problem. -

Intensity of Double-Slit Pattern • Three Or More Slits
• Intensity of double-slit pattern • Three or more slits Practice: Chapter 37, problems 13, 14, 17, 19, 21 Screen at ∞ θ d Path difference Δr = d sin θ zero intensity at d sinθ = (m ± ½ ) λ, m = 0, ± 1, ± 2, … max. intensity at d sinθ = m λ, m = 0, ± 1, ± 2, … 1 Questions: -what is the intensity of the double-slit pattern at an arbitrary position? -What about three slits? four? five? Find the intensity of the double-slit interference pattern as a function of position on the screen. Two waves: E1 = E0 sin(ωt) E2 = E0 sin(ωt + φ) where φ =2π (d sin θ )/λ , and θ gives the position. Steps: Find resultant amplitude, ER ; then intensities obey where I0 is the intensity of each individual wave. 2 Trigonometry: sin a + sin b = 2 cos [(a-b)/2] sin [(a+b)/2] E1 + E2 = E0 [sin(ωt) + sin(ωt + φ)] = 2 E0 cos (φ / 2) cos (ωt + φ / 2)] Resultant amplitude ER = 2 E0 cos (φ / 2) Resultant intensity, (a function of position θ on the screen) I position - fringes are wide, with fuzzy edges - equally spaced (in sin θ) - equal brightness (but we have ignored diffraction) 3 With only one slit open, the intensity in the centre of the screen is 100 W/m 2. With both (identical) slits open together, the intensity at locations of constructive interference will be a) zero b) 100 W/m2 c) 200 W/m2 d) 400 W/m2 How does this compare with shining two laser pointers on the same spot? θ θ sin d = d Δr1 d θ sin d = 2d θ Δr2 sin = 3d Δr3 Total, 4 The total field is where φ = 2π (d sinθ )/λ •When d sinθ = 0, λ , 2λ, .. -

Light and Matter Diffraction from the Unified Viewpoint of Feynman's
European J of Physics Education Volume 8 Issue 2 1309-7202 Arlego & Fanaro Light and Matter Diffraction from the Unified Viewpoint of Feynman’s Sum of All Paths Marcelo Arlego* Maria de los Angeles Fanaro** Universidad Nacional del Centro de la Provincia de Buenos Aires CONICET, Argentine *[email protected] **[email protected] (Received: 05.04.2018, Accepted: 22.05.2017) Abstract In this work, we present a pedagogical strategy to describe the diffraction phenomenon based on a didactic adaptation of the Feynman’s path integrals method, which uses only high school mathematics. The advantage of our approach is that it allows to describe the diffraction in a fully quantum context, where superposition and probabilistic aspects emerge naturally. Our method is based on a time-independent formulation, which allows modelling the phenomenon in geometric terms and trajectories in real space, which is an advantage from the didactic point of view. A distinctive aspect of our work is the description of the series of transformations and didactic transpositions of the fundamental equations that give rise to a common quantum framework for light and matter. This is something that is usually masked by the common use, and that to our knowledge has not been emphasized enough in a unified way. Finally, the role of the superposition of non-classical paths and their didactic potential are briefly mentioned. Keywords: quantum mechanics, light and matter diffraction, Feynman’s Sum of all Paths, high education INTRODUCTION This work promotes the teaching of quantum mechanics at the basic level of secondary school, where the students have not the necessary mathematics to deal with canonical models that uses Schrodinger equation. -

Appendix F. Glossary
Appendix F. Glossary 2DEG 2-dimensional electron gas A/D Analog to digital AAAR American Association for Aerosol Research ADC Analog-digital converter AEM Analytical electron microscopy AFM Atomic force microscope/microscopy AFOSR Air Force Office of Scientific Research AIST (Japan) Agency of Industrial Science and Technology AIST (Japan, MITI) Agency of Industrial Science and Technology AMLCD Active matrix liquid crystal display AMM Amorphous microporous mixed (oxides) AMO Atomic, molecular, and optical AMR Anisotropic magnetoresistance ARO (U.S.) Army Research Office ARPES Angle-resolved photoelectron spectroscopy ASET (Japan) Association of Super-Advanced Electronics Technologies ASTC Australia Science and Technology Council ATP (Japan) Angstrom Technology Partnership ATP Adenosine triphosphate B Magnetic flux density B/H loop Closed figure showing B (magnetic flux density) compared to H (magnetic field strength) in a magnetizable material—also called hysteresis loop bcc Body-centered cubic BMBF (Germany) Ministry of Education, Science, Research, and Technology (formerly called BMFT) BOD-FF Bond-order-dependent force field BRITE/EURAM Basic Research of Industrial Technologies for Europe, European Research on Advanced Materials program CAD Computer-assisted design CAIBE Chemically assisted ion beam etching CBE Chemical beam epitaxy 327 328 Appendix F. Glossary CBED Convergent beam electron diffraction cermet Ceramic/metal composite CIP Cold isostatic press CMOS Complementary metal-oxide semiconductor CMP Chemical mechanical polishing -

A Review of Transmission Electron Microscopy of Quasicrystals—How Are Atoms Arranged?
crystals Review A Review of Transmission Electron Microscopy of Quasicrystals—How Are Atoms Arranged? Ruitao Li 1, Zhong Li 1, Zhili Dong 2,* and Khiam Aik Khor 1,* 1 School of Mechanical & Aerospace Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore; [email protected] (R.L.); [email protected] (Z.L.) 2 School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore * Correspondence: [email protected] (Z.D.); [email protected] (K.A.K.); Tel.: +65-6790-6727 (Z.D.); +65-6592-1816 (K.A.K.) Academic Editor: Enrique Maciá Barber Received: 30 June 2016; Accepted: 15 August 2016; Published: 26 August 2016 Abstract: Quasicrystals (QCs) possess rotational symmetries forbidden in the conventional crystallography and lack translational symmetries. Their atoms are arranged in an ordered but non-periodic way. Transmission electron microscopy (TEM) was the right tool to discover such exotic materials and has always been a main technique in their studies since then. It provides the morphological and crystallographic information and images of real atomic arrangements of QCs. In this review, we summarized the achievements of the study of QCs using TEM, providing intriguing structural details of QCs unveiled by TEM analyses. The main findings on the symmetry, local atomic arrangement and chemical order of QCs are illustrated. Keywords: quasicrystal; transmission electron microscopy; symmetry; atomic arrangement 1. Introduction The revolutionary discovery of an Al–Mn compound with 5-fold symmetry by Shechtman et al. [1] in 1982 unveiled a new family of materials—quasicrystals (QCs), which exhibit the forbidden rotational symmetries in the conventional crystallography. -

The Path Integral Approach to Quantum Mechanics Lecture Notes for Quantum Mechanics IV
The Path Integral approach to Quantum Mechanics Lecture Notes for Quantum Mechanics IV Riccardo Rattazzi May 25, 2009 2 Contents 1 The path integral formalism 5 1.1 Introducingthepathintegrals. 5 1.1.1 Thedoubleslitexperiment . 5 1.1.2 An intuitive approach to the path integral formalism . .. 6 1.1.3 Thepathintegralformulation. 8 1.1.4 From the Schr¨oedinger approach to the path integral . .. 12 1.2 Thepropertiesofthepathintegrals . 14 1.2.1 Pathintegralsandstateevolution . 14 1.2.2 The path integral computation for a free particle . 17 1.3 Pathintegralsasdeterminants . 19 1.3.1 Gaussianintegrals . 19 1.3.2 GaussianPathIntegrals . 20 1.3.3 O(ℏ) corrections to Gaussian approximation . 22 1.3.4 Quadratic lagrangians and the harmonic oscillator . .. 23 1.4 Operatormatrixelements . 27 1.4.1 Thetime-orderedproductofoperators . 27 2 Functional and Euclidean methods 31 2.1 Functionalmethod .......................... 31 2.2 EuclideanPathIntegral . 32 2.2.1 Statisticalmechanics. 34 2.3 Perturbationtheory . .. .. .. .. .. .. .. .. .. .. 35 2.3.1 Euclidean n-pointcorrelators . 35 2.3.2 Thermal n-pointcorrelators. 36 2.3.3 Euclidean correlators by functional derivatives . ... 38 0 0 2.3.4 Computing KE[J] and Z [J] ................ 39 2.3.5 Free n-pointcorrelators . 41 2.3.6 The anharmonic oscillator and Feynman diagrams . 43 3 The semiclassical approximation 49 3.1 Thesemiclassicalpropagator . 50 3.1.1 VanVleck-Pauli-Morette formula . 54 3.1.2 MathematicalAppendix1 . 56 3.1.3 MathematicalAppendix2 . 56 3.2 Thefixedenergypropagator. 57 3.2.1 General properties of the fixed energy propagator . 57 3.2.2 Semiclassical computation of K(E)............. 61 3.2.3 Two applications: reflection and tunneling through a barrier 64 3 4 CONTENTS 3.2.4 On the phase of the prefactor of K(xf ,tf ; xi,ti) .... -
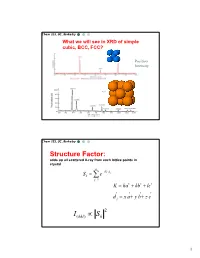
Simple Cubic Lattice
Chem 253, UC, Berkeley What we will see in XRD of simple cubic, BCC, FCC? Position Intensity Chem 253, UC, Berkeley Structure Factor: adds up all scattered X-ray from each lattice points in crystal n iKd j Sk e j1 K ha kb lc d j x a y b z c 2 I(hkl) Sk 1 Chem 253, UC, Berkeley X-ray scattered from each primitive cell interfere constructively when: eiKR 1 2d sin n For n-atom basis: sum up the X-ray scattered from the whole basis Chem 253, UC, Berkeley ' k d k d di R j ' K k k Phase difference: K (di d j ) The amplitude of the two rays differ: eiK(di d j ) 2 Chem 253, UC, Berkeley The amplitude of the rays scattered at d1, d2, d3…. are in the ratios : eiKd j The net ray scattered by the entire cell: n iKd j Sk e j1 2 I(hkl) Sk Chem 253, UC, Berkeley For simple cubic: (0,0,0) iK0 Sk e 1 3 Chem 253, UC, Berkeley For BCC: (0,0,0), (1/2, ½, ½)…. Two point basis 1 2 iK ( x y z ) iKd j iK0 2 Sk e e e j1 1 ei (hk l) 1 (1)hkl S=2, when h+k+l even S=0, when h+k+l odd, systematical absence Chem 253, UC, Berkeley For BCC: (0,0,0), (1/2, ½, ½)…. Two point basis S=2, when h+k+l even S=0, when h+k+l odd, systematical absence (100): destructive (200): constructive 4 Chem 253, UC, Berkeley Observable diffraction peaks h2 k 2 l 2 Ratio SC: 1,2,3,4,5,6,8,9,10,11,12. -

Development of Rotation Electron Diffraction As a Fully Automated And
Bin Wang Development of rotation electron Development of rotation electron diffraction as a fully automated and accurate method for structure determination and accurate automated as a fully diffraction electron of rotation Development diffraction as a fully automated and accurate method for structure determination Bin Wang Bin Wang was born in Shanghai, China. He received his B.Sc in chemistry from Fudan University in China in 2013, and M.Sc in material chemistry from Cornell University in the US in 2015. His research mainly focused on method development for TEM. ISBN 978-91-7797-646-2 Department of Materials and Environmental Chemistry Doctoral Thesis in Inorganic Chemistry at Stockholm University, Sweden 2019 Development of rotation electron diffraction as a fully automated and accurate method for structure determination Bin Wang Academic dissertation for the Degree of Doctor of Philosophy in Inorganic Chemistry at Stockholm University to be publicly defended on Monday 10 June 2019 at 13.00 in Magnélisalen, Kemiska övningslaboratoriet, Svante Arrhenius väg 16 B. Abstract Over the past decade, electron diffraction methods have aroused more and more interest for micro-crystal structure determination. Compared to traditional X-ray diffraction, electron diffraction breaks the size limitation of the crystals studied, but at the same time it also suffers from much stronger dynamical effects. While X-ray crystallography has been almost thoroughly developed, electron crystallography is still under active development. To be able to perform electron diffraction experiments, adequate skills for using a TEM are usually required, which makes ED experiments less accessible to average users than X-ray diffraction. Moreover, the relatively poor data statistics from ED data prevented electron crystallography from being widely accepted in the crystallography community. -

Diffraction-Free Beams in Fractional Schrödinger Equation Yiqi Zhang1, Hua Zhong1, Milivoj R
www.nature.com/scientificreports OPEN Diffraction-free beams in fractional Schrödinger equation Yiqi Zhang1, Hua Zhong1, Milivoj R. Belić2, Noor Ahmed1, Yanpeng Zhang1 & Min Xiao3,4 We investigate the propagation of one-dimensional and two-dimensional (1D, 2D) Gaussian beams in Received: 14 December 2015 the fractional Schrödinger equation (FSE) without a potential, analytically and numerically. Without Accepted: 11 March 2016 chirp, a 1D Gaussian beam splits into two nondiffracting Gaussian beams during propagation, while a 2D Published: 21 April 2016 Gaussian beam undergoes conical diffraction. When a Gaussian beam carries linear chirp, the 1D beam deflects along the trajectoriesz = ±2(x − x0), which are independent of the chirp. In the case of 2D Gaussian beam, the propagation is also deflected, but the trajectories align along the diffraction cone z =+2 xy22 and the direction is determined by the chirp. Both 1D and 2D Gaussian beams are diffractionless and display uniform propagation. The nondiffracting property discovered in this model applies to other beams as well. Based on the nondiffracting and splitting properties, we introduce the Talbot effect of diffractionless beams in FSE. Fractional effects, such as fractional quantum Hall effect1, fractional Talbot effect2, fractional Josephson effect3 and other effects coming from the fractional Schrödinger equation (FSE)4, introduce seminal phenomena in and open new areas of physics. FSE, developed by Laskin5–7, is a generalization of the Schrödinger equation (SE) that contains fractional Laplacian instead of the usual one, which describes the behavior of particles with fractional spin8. This generalization produces nonlocal features in the wave function. In the last decade, research on FSE was very intensive9–17. -

The Doubling of the Degrees of Freedom in Quantum Dissipative Systems, and the Semantic Information Notion and Measure in Biosemiotics †
Proceedings The Doubling of the Degrees of Freedom in Quantum Dissipative Systems, and the Semantic † Information Notion and Measure in Biosemiotics Gianfranco Basti 1,*, Antonio Capolupo 2,3 and GiuseppeVitiello 2 1 Faculty of Philosophy, Pontifical Lateran University, 00120 Vatican City, Italy 2 Department of Physics “E:R. Caianiello”, University of Salerno, Via Giovanni Paolo II, 132, 84084 Fisciano (SA), Italy; [email protected] (A.C.); [email protected] (G.V.) 3 Istituto Nazionale di Fisica Nucleare (INFN), Gruppo Collegato di Salerno, Via Giovanni Paolo II, 132, 84084 Fisciano (SA), Italy * Correspondence: [email protected]; Tel.: +39-339-576-0314 † Conference Theoretical Information Studies (TIS), Berkeley, CA, USA, 2–6 June 2019. Published: 11 May 2020 Keywords: semantic information; non-equilibrium thermodynamics; quantum field theory 1. The Semantic Information Issue and Non-Equilibrium Thermodynamics In the recent history of the effort for defining a suitable notion and measure of semantic information, starting points are: the “syntactic” notion and measure of information H of Claude Shannon in the mathematical theory of communications (MTC) [1]; and the “semantic” notion and measure of information content or informativeness of Rudolph Carnap and Yehoshua Bar-Hillel [2], based on Carnap’s theory of intensional modal logic [3]. The relationship between H and the notion and measure of entropy S in Gibbs’ statistical mechanics (SM) and then in Boltzmann’s thermodynamics is a classical one, because they share the same mathematical form. We emphasize that Shannon’s information measure is the information associated to an event in quantum mechanics (QM) [4]. Indeed, in the classical limit, i.e., whenever the classical notion of probability applies, S is equivalent to the QM definition of entropy given by John Von Neumann. -

Chapter 14 Interference and Diffraction
Chapter 14 Interference and Diffraction 14.1 Superposition of Waves.................................................................................... 14-2 14.2 Young’s Double-Slit Experiment ..................................................................... 14-4 Example 14.1: Double-Slit Experiment................................................................ 14-7 14.3 Intensity Distribution ........................................................................................ 14-8 Example 14.2: Intensity of Three-Slit Interference ............................................ 14-11 14.4 Diffraction....................................................................................................... 14-13 14.5 Single-Slit Diffraction..................................................................................... 14-13 Example 14.3: Single-Slit Diffraction ................................................................ 14-15 14.6 Intensity of Single-Slit Diffraction ................................................................. 14-16 14.7 Intensity of Double-Slit Diffraction Patterns.................................................. 14-19 14.8 Diffraction Grating ......................................................................................... 14-20 14.9 Summary......................................................................................................... 14-22 14.10 Appendix: Computing the Total Electric Field............................................. 14-23 14.11 Solved Problems .......................................................................................... -

Three-Dimensional Electron Crystallography of Protein Microcrystals Dan Shi†, Brent L Nannenga†, Matthew G Iadanza†, Tamir Gonen*
RESEARCH ARTICLE elife.elifesciences.org Three-dimensional electron crystallography of protein microcrystals Dan Shi†, Brent L Nannenga†, Matthew G Iadanza†, Tamir Gonen* Janelia Farm Research Campus, Howard Hughes Medical Institute, Ashburn, United States Abstract We demonstrate that it is feasible to determine high-resolution protein structures by electron crystallography of three-dimensional crystals in an electron cryo-microscope (CryoEM). Lysozyme microcrystals were frozen on an electron microscopy grid, and electron diffraction data collected to 1.7 Å resolution. We developed a data collection protocol to collect a full-tilt series in electron diffraction to atomic resolution. A single tilt series contains up to 90 individual diffraction patterns collected from a single crystal with tilt angle increment of 0.1–1° and a total accumulated electron dose less than 10 electrons per angstrom squared. We indexed the data from three crystals and used them for structure determination of lysozyme by molecular replacement followed by crystallographic refinement to 2.9 Å resolution. This proof of principle paves the way for the implementation of a new technique, which we name ‘MicroED’, that may have wide applicability in structural biology. DOI: 10.7554/eLife.01345.001 Introduction X-ray crystallography depends on large and well-ordered crystals for diffraction studies. Crystals are *For correspondence: gonent@ solids composed of repeated structural motifs in a three-dimensional lattice (hereafter called ‘3D janelia.hhmi.org crystals’). The periodic structure of the crystalline solid acts as a diffraction grating to scatter the †These authors contributed X-rays. For every elastic scattering event that contributes to a diffraction pattern there are ∼10 inelastic equally to this work events that cause beam damage (Henderson, 1995).