Reactive Molecular Dynamics Study of the Thermal Decomposition of Phenolic Resins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Olaseni Sode
Olaseni Sode Work Address: 5735 S. Ellis Ave. [email protected] Searle 231 +1 (314) 856-2373 The University of Chicago Chicago, IL 60615 Home Address: 1119 E Hyde Park Blvd [email protected] Apt. 3E +1 (314) 856-2373 Chicago, IL 60615 http://www.olaseniosode.com Education University of Illinois at Urbana-Champaign Urbana, IL Ph.D. in Chemistry September 2012 – Supervisor: Professor So Hirata – Thesis title: A Theoretical Study of Molecular Crystals – Transferred from the University of Florida in 2010 with Prof. Hirata Morehouse College Atlanta, GA B.S. & B.A. in Chemistry and French December 2006 – Studied at the Université de Nantes, Nantes, France (2004-2005) Research Experience The University of Chicago Chicago, IL Postdoctoral Research – research advisor: Dr. Gregory A. Voth 2012–present – Studied proposed proton transport minimum free-energy pathways in [FeFe]-hydrogenase via molecular dynamics simulations and the multistate empirical valence bond technique. – Collaborated to develop the multiscale string method to accelerate refinement of chemical reaction pathways of adenosine triphosphate (ATP) hydrolysis in globular and filamentous actin proteins. University of California, San Diego La Jolla, CA Visiting Scholar – research advisor: Dr. Francesco Paesani 2014, 2015 – Developed and implemented a reactive molecular dynamics scheme for condensed phase systems of the hydrated hydronium ions. University of Illinois at Urbana-Champaign Urbana, IL Doctoral Research – research advisor: Dr. So Hirata 2010-2012 – Characterized the vibrational structure of extended systems including infrared and Raman-active vibrations, as well as phonon dispersions and densities of states in the first Brillouin zone. – Developed zero-point vibrational energy analysis scheme for molecular clusters using a fragmented, local basis scheme. -

Pysimm: a Python Package for Simulation of Molecular Systems
SoftwareX 6 (2016) 7–12 Contents lists available at ScienceDirect SoftwareX journal homepage: www.elsevier.com/locate/softx pysimm: A python package for simulation of molecular systems Michael E. Fortunato a, Coray M. Colina a,b,∗ a Department of Chemistry, University of Florida, Gainesville, FL 32611, United States b Department of Materials Science and Engineering and Nuclear Engineering, University of Florida, Gainesville, FL 32611, United States article info a b s t r a c t Article history: In this work, we present pysimm, a python package designed to facilitate structure generation, simulation, Received 15 August 2016 and modification of molecular systems. pysimm provides a collection of simulation tools and smooth Received in revised form integration with highly optimized third party software. Abstraction layers enable a standardized 1 December 2016 methodology to assign various force field models to molecular systems and perform simple simulations. Accepted 5 December 2016 These features have allowed pysimm to aid the rapid development of new applications specifically in the area of amorphous polymer simulations. Keywords: ' 2016 The Authors. Published by Elsevier B.V. Amorphous polymers Molecular simulation This is an open access article under the CC BY license Python (http://creativecommons.org/licenses/by/4.0/). Code metadata Current code version v0.1 Permanent link to code/repository used for this code version https://github.com/ElsevierSoftwareX/SOFTX-D-16-00070 Legal Code License MIT Code versioning system used git Software code languages, tools, and services used python2.7 Compilation requirements, operating environments & Linux dependencies If available Link to developer documentation/manual http://pysimm.org/documentation/ Support email for questions [email protected] 1. -

A Study on Cheminformatics and Its Applications on Modern Drug Discovery
Available online at www.sciencedirect.com Procedia Engineering 38 ( 2012 ) 1264 – 1275 Internatio na l Conference on Modeling Optimisatio n and Computing (ICMOC 2012) A Study on Cheminformatics and its Applications on Modern Drug Discovery B.Firdaus Begama and Dr. J.Satheesh Kumarb aResearch Scholar, Bharathiar University, Coimbatore, India, [email protected] bAssistant Professor, Bharathiar University, Coimbatore, India, [email protected] Abstract Discovering drugs to a disease is still a challenging task for medical researchers due to the complex structures of biomolecules which are responsible for disease such as AIDS, Cancer, Autism, Alzimear etc. Design and development of new efficient anti-drugs for the disease without any side effects are becoming mandatory in the recent history of human life cycle due to changes in various factors which includes food habit, environmental and migration in human life style. Cheminformaticds deals with discovering drugs based in modern drug discovery techniques which in turn rectifies complex issues in traditional drug discovery system. Cheminformatics tools, helps medical chemist for better understanding of complex structures of chemical compounds. Cheminformatics is a new emerging interdisciplinary field which primarily aims to discover Novel Chemical Entities [NCE] which ultimately results in design of new molecule [chemical data]. It also plays an important role for collecting, storing and analysing the chemical data. This paper focuses on cheminformatics and its applications on drug discovery and modern drug discovery techniques which helps chemist and medical researchers for finding solution to the complex disease. © 2012 Published by Elsevier Ltd. Selection and/or peer-review under responsibility of Noorul Islam Centre for Higher Education. -
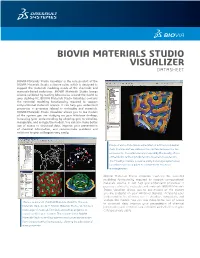
Biovia Materials Studio Visualizer Datasheet
BIOVIA MATERIALS STUDIO VISUALIZER DATASHEET BIOVIA Materials Studio Visualizer is the core product of the BIOVIA Materials Studio software suite, which is designed to support the materials modeling needs of the chemicals and materials-based industries. BIOVIA Materials Studio brings science validated by leading laboratories around the world to your desktop PC. BIOVIA Materials Studio Visualizer contains the essential modeling functionality required to support computational materials science. It can help you understand properties or processes related to molecules and materials. BIOVIA Materials Studio Visualizer allows you to see models of the system you are studying on your Windows desktop, increasing your understanding by allowing you to visualize, manipulate, and analyze the models. You can also make better use of access to structural data, improve your presentation of chemical information, and communicate problems and solutions to your colleagues very easily. Image of early-stage phase segregation in a diblock copolymer melt. The blue surface indicates the interface between the two components. The volume is colormapped by the density of one of the blocks, red being high density, blue being low-density. The MesoDyn module is used to study these large systems over long-times such as required to observe these structural rearrangements. BIOVIA Materials Studio Visualizer contains the essential modeling functionality required to support computational materials science. It can help you understand properties or processes related to molecules and materials. BIOVIA Materials Studio Visualizer allows you to see models of the system you are studying on your Windows desktop, increasing your understanding by allowing you to visualize, manipulate, and analyze the models. -

Download Author Version (PDF)
PCCP Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/pccp Page 1 of 11 PhysicalPlease Chemistry do not adjust Chemical margins Physics PCCP PAPER Effect of nanosize on surface properties of NiO nanoparticles for adsorption of Quinolin-65 ab a a Received 00th January 20xx, Nedal N. Marei, Nashaat N. Nassar* and Gerardo Vitale Accepted 00th January 20xx Using Quinolin-65 (Q-65) as a model-adsorbing compound for polar heavy hydrocarbons, the nanosize effect of NiO Manuscript DOI: 10.1039/x0xx00000x nanoparticles on adsorption of Q-65 was investigated. Different-sized NiO nanoparticles with sizes between 5 and 80 nm were prepared by controlled thermal dehydroxylation of Ni(OH)2. -

Supporting Information
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2015 Supporting Information A Single Crystalline Porphyrinic Titanium Metal−Organic Framework Shuai Yuana†, Tian-Fu Liua†, Dawei Fenga, Jian Tiana, Kecheng Wanga, Junsheng Qina, a a a a b Qiang Zhang , Ying-Pin Chen , Mathieu Bosch , Lanfang Zou , Simon J. Teat, Scott J. c a Dalgarno and Hong-Cai Zhou * a Department of Chemistry, Texas A&M University, College Station, Texas 77842-3012, USA b Advanced Light Source, Lawrence Berkeley National Laboratory Berkeley, CA 94720, USA c Institute of Chemical Sciences, Heriot-Watt University Riccarton, Edinburgh EH14 4AS, U.K. † Equal contribution to this work *To whom correspondence should be addressed. Email: [email protected] Tel: +1 (979) 845-4034; Fax: +1 (979) 845-1595 S1 Contents S1. Ligand Synthesis..............................................................................................................3 S2. Syntheses of PCN-22.......................................................................................................5 S3. X-ray Crystallography .....................................................................................................6 S4. Topological Analyses ......................................................................................................9 S5. N2 Sorption Isotherm .....................................................................................................10 S6. Simulation of the Accessible Surface Area ...................................................................12 -

Dmol Guide to Select a Dmol3 Task 1
DMOL3 GUIDE MATERIALS STUDIO 8.0 Copyright Notice ©2014 Dassault Systèmes. All rights reserved. 3DEXPERIENCE, the Compass icon and the 3DS logo, CATIA, SOLIDWORKS, ENOVIA, DELMIA, SIMULIA, GEOVIA, EXALEAD, 3D VIA, BIOVIA and NETVIBES are commercial trademarks or registered trademarks of Dassault Systèmes or its subsidiaries in the U.S. and/or other countries. All other trademarks are owned by their respective owners. Use of any Dassault Systèmes or its subsidiaries trademarks is subject to their express written approval. Acknowledgments and References To print photographs or files of computational results (figures and/or data) obtained using BIOVIA software, acknowledge the source in an appropriate format. For example: "Computational results obtained using software programs from Dassault Systèmes Biovia Corp.. The ab initio calculations were performed with the DMol3 program, and graphical displays generated with Materials Studio." BIOVIA may grant permission to republish or reprint its copyrighted materials. Requests should be submitted to BIOVIA Support, either through electronic mail to [email protected], or in writing to: BIOVIA Support 5005 Wateridge Vista Drive, San Diego, CA 92121 USA Contents DMol3 1 Setting up a molecular dynamics calculation20 Introduction 1 Choosing an ensemble 21 Further Information 1 Defining the time step 21 Tasks in DMol3 2 Defining the thermostat control 21 Energy 3 Constraints during dynamics 21 Setting up the calculation 3 Setting up a transition state calculation 22 Dynamics 4 Which method to use? -

How to Modify LAMMPS: from the Prospective of a Particle Method Researcher
chemengineering Article How to Modify LAMMPS: From the Prospective of a Particle Method Researcher Andrea Albano 1,* , Eve le Guillou 2, Antoine Danzé 2, Irene Moulitsas 2 , Iwan H. Sahputra 1,3, Amin Rahmat 1, Carlos Alberto Duque-Daza 1,4, Xiaocheng Shang 5 , Khai Ching Ng 6, Mostapha Ariane 7 and Alessio Alexiadis 1,* 1 School of Chemical Engineering, University of Birmingham, Birmingham B15 2TT, UK; [email protected] (I.H.S.); [email protected] (A.R.); [email protected] (C.A.D.-D.) 2 Centre for Computational Engineering Sciences, Cranfield University, Bedford MK43 0AL, UK; Eve.M.Le-Guillou@cranfield.ac.uk (E.l.G.); A.Danze@cranfield.ac.uk (A.D.); i.moulitsas@cranfield.ac.uk (I.M.) 3 Industrial Engineering Department, Petra Christian University, Surabaya 60236, Indonesia 4 Department of Mechanical and Mechatronic Engineering, Universidad Nacional de Colombia, Bogotá 111321, Colombia 5 School of Mathematics, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK; [email protected] 6 Department of Mechanical, Materials and Manufacturing Engineering, University of Nottingham Malaysia, Jalan Broga, Semenyih 43500, Malaysia; [email protected] 7 Department of Materials and Engineering, Sayens-University of Burgundy, 21000 Dijon, France; [email protected] * Correspondence: [email protected] or [email protected] (A.A.); [email protected] (A.A.) Citation: Albano, A.; le Guillou, E.; Danzé, A.; Moulitsas, I.; Sahputra, Abstract: LAMMPS is a powerful simulator originally developed for molecular dynamics that, today, I.H.; Rahmat, A.; Duque-Daza, C.A.; also accounts for other particle-based algorithms such as DEM, SPH, or Peridynamics. -

Preparing and Analyzing Large Molecular Simulations With
Preparing and Analyzing Large Molecular Simulations with VMD John Stone, Senior Research Programmer NIH Center for Macromolecular Modeling and Bioinformatics, University of Illinois at Urbana-Champaign VMD Tutorials Home Page • http://www.ks.uiuc.edu/Training/Tutorials/ – Main VMD tutorial – VMD images and movies tutorial – QwikMD simulation preparation and analysis plugin – Structure check – VMD quantum chemistry visualization tutorial – Visualization and analysis of CPMD data with VMD – Parameterizing small molecules using ffTK Overview • Introduction • Data model • Visualization • Scripting and analytical features • Trajectory analysis and visualization • High fidelity ray tracing • Plugins and user-extensibility • Large system analysis and visualization VMD – “Visual Molecular Dynamics” • 100,000 active users worldwide • Visualization and analysis of: – Molecular dynamics simulations – Lattice cell simulations – Quantum chemistry calculations – Cryo-EM densities, volumetric data • User extensible scripting and plugins • http://www.ks.uiuc.edu/Research/vmd/ Cell-Scale Modeling MD Simulation VMD – “Visual Molecular Dynamics” • Unique capabilities: • Visualization and analysis of: – Trajectories are fundamental to VMD – Molecular dynamics simulations – Support for very large systems, – “Particle” systems and whole cells now reaching billions of particles – Cryo-EM densities, volumetric data – Extensive GPU acceleration – Quantum chemistry calculations – Parallel analysis/visualization with MPI – Sequence information MD Simulations Cell-Scale -

Biomolecular Simulation Data Management In
BIOMOLECULAR SIMULATION DATA MANAGEMENT IN HETEROGENEOUS ENVIRONMENTS Julien Charles Victor Thibault A dissertation submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Biomedical Informatics The University of Utah December 2014 Copyright © Julien Charles Victor Thibault 2014 All Rights Reserved The University of Utah Graduate School STATEMENT OF DISSERTATION APPROVAL The dissertation of Julien Charles Victor Thibault has been approved by the following supervisory committee members: Julio Cesar Facelli , Chair 4/2/2014___ Date Approved Thomas E. Cheatham , Member 3/31/2014___ Date Approved Karen Eilbeck , Member 4/3/2014___ Date Approved Lewis J. Frey _ , Member 4/2/2014___ Date Approved Scott P. Narus , Member 4/4/2014___ Date Approved And by Wendy W. Chapman , Chair of the Department of Biomedical Informatics and by David B. Kieda, Dean of The Graduate School. ABSTRACT Over 40 years ago, the first computer simulation of a protein was reported: the atomic motions of a 58 amino acid protein were simulated for few picoseconds. With today’s supercomputers, simulations of large biomolecular systems with hundreds of thousands of atoms can reach biologically significant timescales. Through dynamics information biomolecular simulations can provide new insights into molecular structure and function to support the development of new drugs or therapies. While the recent advances in high-performance computing hardware and computational methods have enabled scientists to run longer simulations, they also created new challenges for data management. Investigators need to use local and national resources to run these simulations and store their output, which can reach terabytes of data on disk. -

Molecular Dynamics Simulations of Molecular Diffusion Equilibrium and Breakdown Mechanism of Oil-Impregnated Pressboard with Water Impurity
polymers Article Molecular Dynamics Simulations of Molecular Diffusion Equilibrium and Breakdown Mechanism of Oil-Impregnated Pressboard with Water Impurity Yi Guan 1,2, Ming-He Chi 1,2, Wei-Feng Sun 1,2,*, Qing-Guo Chen 1,2 and Xin-Lao Wei 1,2 1 Heilongjiang Provincial Key Laboratory of Dielectric Engineering, School of Electrical and Electronic Engineering, Harbin University of Science and Technology, Harbin 150080, China; [email protected] (Y.G.); [email protected] (M.-H.C.); [email protected] (Q.-G.C.); [email protected] (X.-L.W.) 2 Key Laboratory of Engineering Dielectrics and Its Application, Ministry of Education, Harbin University of Science and Technology, Harbin 150080, China * Correspondence: [email protected]; Tel.: +86-15846592798 Received: 30 October 2018; Accepted: 15 November 2018; Published: 16 November 2018 Abstract: The water molecule migration and aggregation behaviors in oil-impregnated pressboard are investigated by molecular dynamics simulations in combination with Monte Carlo molecular simulation technique. The free energy and phase diagram of H2O-dodecylbenzene (DDB) and H2O-cellulose mixtures are calculated by Monte Carlo technique combined with the modified Flory-Huggins model, demonstrating that H2O molecules can hardly dissolved with infinitesimal content in cellulose system at temperature lower than 650 K, based on which the oil/cellulose layered structure with water impurity representing three-phase coexistence in oil-impregnated pressboard are modeled and performed for molecular dynamics. The molecular dynamics of H2O/DDB/cellulose three-phase mixture simulating oil-paper insulating system with H2O impurity indicates that DDB molecules can thermally intrude into the cellulose-water interface so as to separate the water phase and cellulose fiber. -

What's New in Biovia Materials Studio 2020
WHAT’S NEW IN BIOVIA MATERIALS STUDIO 2020 Datasheet BIOVIA Materials Studio 2020 is the latest release of BIOVIA’s predictive science tools for chemistry and materials science research. Materials Studio empowers researchers to understand the relationships between a material’s mo- lecular or crystal structure and its properties in order to make more informed decisions about materials research and development. More often than not materials performance is influenced by phenomena at multiple scales. Scientists using Materials Studio 2020 have an extensive suite of world class solvers and parameter sets operating from atoms to microscale for simulating more materials and more properties than ever before. Furthermore, the predicted properties can now be used in multi-physics modeling and in systems modeling software such as SIMULIA Abaqus and CATIA Dymola to predict macroscopic behaviors. In this way multiscale simulations can be used to solve some of the toughest pro- blems in materials design and product optimization. BETTER MATERIALS - BETTER BATTERIES Safe, fast charging batteries with high energy density and long life are urgently needed for a host of applications - not least for the electrification of all modes of transportation as an alternative to fossil fuel energy sources. Battery design relies on a complex interplay between thermal, mechanical and chemical processes from the smallest scales of the material (electronic structure) through to the geometry of the battery cell and pack design. Improvements to the component materials used in batteries and capacitors are fundamental to providing the advances in performance needed. Materials Studio provides new functionality to enable the simula- tion of key materials parameters for both liquid electrolytes and electrode components.