Metric System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dimensional Analysis and the Theory of Natural Units
LIBRARY TECHNICAL REPORT SECTION SCHOOL NAVAL POSTGRADUATE MONTEREY, CALIFORNIA 93940 NPS-57Gn71101A NAVAL POSTGRADUATE SCHOOL Monterey, California DIMENSIONAL ANALYSIS AND THE THEORY OF NATURAL UNITS "by T. H. Gawain, D.Sc. October 1971 lllp FEDDOCS public This document has been approved for D 208.14/2:NPS-57GN71101A unlimited release and sale; i^ distribution is NAVAL POSTGRADUATE SCHOOL Monterey, California Rear Admiral A. S. Goodfellow, Jr., USN M. U. Clauser Superintendent Provost ABSTRACT: This monograph has been prepared as a text on dimensional analysis for students of Aeronautics at this School. It develops the subject from a viewpoint which is inadequately treated in most standard texts hut which the author's experience has shown to be valuable to students and professionals alike. The analysis treats two types of consistent units, namely, fixed units and natural units. Fixed units include those encountered in the various familiar English and metric systems. Natural units are not fixed in magnitude once and for all but depend on certain physical reference parameters which change with the problem under consideration. Detailed rules are given for the orderly choice of such dimensional reference parameters and for their use in various applications. It is shown that when transformed into natural units, all physical quantities are reduced to dimensionless form. The dimension- less parameters of the well known Pi Theorem are shown to be in this category. An important corollary is proved, namely that any valid physical equation remains valid if all dimensional quantities in the equation be replaced by their dimensionless counterparts in any consistent system of natural units. -

Chapter 5 Dimensional Analysis and Similarity
Chapter 5 Dimensional Analysis and Similarity Motivation. In this chapter we discuss the planning, presentation, and interpretation of experimental data. We shall try to convince you that such data are best presented in dimensionless form. Experiments which might result in tables of output, or even mul- tiple volumes of tables, might be reduced to a single set of curves—or even a single curve—when suitably nondimensionalized. The technique for doing this is dimensional analysis. Chapter 3 presented gross control-volume balances of mass, momentum, and en- ergy which led to estimates of global parameters: mass flow, force, torque, total heat transfer. Chapter 4 presented infinitesimal balances which led to the basic partial dif- ferential equations of fluid flow and some particular solutions. These two chapters cov- ered analytical techniques, which are limited to fairly simple geometries and well- defined boundary conditions. Probably one-third of fluid-flow problems can be attacked in this analytical or theoretical manner. The other two-thirds of all fluid problems are too complex, both geometrically and physically, to be solved analytically. They must be tested by experiment. Their behav- ior is reported as experimental data. Such data are much more useful if they are ex- pressed in compact, economic form. Graphs are especially useful, since tabulated data cannot be absorbed, nor can the trends and rates of change be observed, by most en- gineering eyes. These are the motivations for dimensional analysis. The technique is traditional in fluid mechanics and is useful in all engineering and physical sciences, with notable uses also seen in the biological and social sciences. -
Metric Conversion Worksheet – Solutions
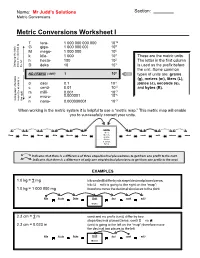
Name: Mr Judd’s Solutions Section: Metric Conversions Metric Conversions Worksheet I T tera- 1 000 000 000 000 1012 9 G giga- 1 000 000 000 10 M mega- 1 000 000 106 k kilo- 1 000 103 These are the metric units. h hecto- 100 102 The letter in the first column 1 Going table up the movethe decimal to theleft D deka 10 10 is used as the prefix before the unit. Some common NO PREFIX ( UNIT) 1 100 types of units are: grams (g), meters (m), liters (L), d deci 0.1 10-1 joules (J), seconds (s), c centi- 0.01 10-2 and bytes (B). m milli- 0.001 10-3 -6 μ micro- 0.000001 10 Goingtable the down movethe decimal to theright n nano- 0.000000001 10-9 When working in the metric system it is helpful to use a “metric map.” This metric map will enable you to successfully convert your units. Units Liters Tera Giga Mega Kilo Hecto Deka Meters deci centi milli micro nano Grams Joules Seconds Bytes indicates that there is a difference of three steps/decimal places/zeros to get from one prefix to the next. indicates that there is a difference of only one step/decimal place/zero to get from one prefix to the next. EXAMPLES 1.0 kg = ? mg 1.0 kg = 1 000 000 mg Kilo Hecto Deka Unit deci centi milli Grams 2.3 cm = ? m 2.3 cm = 0.023 m (unit) is going to the left on the “map”; therefore move the decimal two places to the left Kilo Hecto Deka Unit deci centi milli Meters From To Difference Move decimal in zeros/ left or right? prefix unit prefix unit decimal places/steps km kilo Meters m no prefix Meters 3 right Mg mega grams Gg giga grams 3 left mL milli Litres L no prefix Litres 3 left kJ kilo Joules mJ milli Joules 6 right μs micro seconds ms milli seconds 3 left B no prefix bytes kB kilo bytes 3 left cm centi metre mm milli metre 1 right cL centi Litres μL micro Litres 4 right PROBLEMS 1. -

Three-Dimensional Analysis of the Hot-Spot Fuel Temperature in Pebble Bed and Prismatic Modular Reactors
Transactions of the Korean Nuclear Society Spring Meeting Chuncheon, Korea, May 25-26 2006 Three-Dimensional Analysis of the Hot-Spot Fuel Temperature in Pebble Bed and Prismatic Modular Reactors W. K. In,a S. W. Lee,a H. S. Lim,a W. J. Lee a a Korea Atomic Energy Research Institute, P. O. Box 105, Yuseong, Daejeon, Korea, 305-600, [email protected] 1. Introduction thousands of fuel particles. The pebble diameter is 60mm High temperature gas-cooled reactors(HTGR) have with a 5mm unfueled layer. Unstaggered and 2-D been reviewed as potential sources for future energy needs, staggered arrays of 3x3 pebbles as shown in Fig. 1 are particularly for a hydrogen production. Among the simulated to predict the temperature distribution in a hot- HTGRs, the pebble bed reactor(PBR) and a prismatic spot pebble core. Total number of nodes is 1,220,000 and modular reactor(PMR) are considered as the nuclear heat 1,060,000 for the unstaggered and staggered models, source in Korea’s nuclear hydrogen development and respectively. demonstration project. PBR uses coated fuel particles The GT-MHR(PMR) reactor core is loaded with an embedded in spherical graphite fuel pebbles. The fuel annular stack of hexahedral prismatic fuel assemblies, pebbles flow down through the core during an operation. which form 102 columns consisting of 10 fuel blocks PMR uses graphite fuel blocks which contain cylindrical stacked axially in each column. Each fuel block is a fuel compacts consisting of the fuel particles. The fuel triangular array of a fuel compact channel, a coolant blocks also contain coolant passages and locations for channel and a channel for a control rod. -

Units of Measurement and Dimensional Analysis
Measurements and Dimensional Analysis POGIL ACTIVITY.2 Name ________________________________________ POGIL ACTIVITY 2 Units of Measurement and Dimensional Analysis A. Units of Measurement- The SI System and Metric System here are myriad units for measurement. For example, length is reported in miles T or kilometers; mass is measured in pounds or kilograms and volume can be given in gallons or liters. To avoid confusion, scientists have adopted an international system of units commonly known as the SI System. Standard units are called base units. Table A1. SI System (Systéme Internationale d’Unités) Measurement Base Unit Symbol mass gram g length meter m volume liter L temperature Kelvin K time second s energy joule j pressure atmosphere atm 7 Measurements and Dimensional Analysis POGIL. ACTIVITY.2 Name ________________________________________ The metric system combines the powers of ten and the base units from the SI System. Powers of ten are used to derive larger and smaller units, multiples of the base unit. Multiples of the base units are defined by a prefix. When metric units are attached to a number, the letter symbol is used to abbreviate the prefix and the unit. For example, 2.2 kilograms would be reported as 2.2 kg. Plural units, i.e., (kgs) are incorrect. Table A2. Common Metric Units Power Decimal Prefix Name of metric unit (and symbol) Of Ten equivalent (symbol) length volume mass 103 1000 kilo (k) kilometer (km) B kilogram (kg) Base 100 1 meter (m) Liter (L) gram (g) Unit 10-1 0.1 deci (d) A deciliter (dL) D 10-2 0.01 centi (c) centimeter (cm) C E 10-3 0.001 milli (m) millimeter (mm) milliliter (mL) milligram (mg) 10-6 0.000 001 micro () micrometer (m) microliter (L) microgram (g) Critical Thinking Questions CTQ 1 Consult Table A2. -

Metric System Conversion Factors1 J
AGR39 Metric System Conversion Factors1 J. Bryan Unruh, Barry J. Brecke, and Ramon G. Leon-Gonzalez2 Area Equivalents 1 Hectare (ha) 2 1 Acre (A) = 10,000 square meters (m ) 2 = 100 are (a) = 43,560 square feet (ft ) = 2.471 acres (A) = 4,840 square yards (yd2) = 0.405 hectares (ha) 1 Square Foot (ft) = 160 square rods (rd2) 2 = 4,047 square meters (m2) = 144 square inches (in ) = 929.03 square centimeters (cm2) 2 1 Acre-inch (ac-in) = 0.0929 square meters (m ) 3 = 102.8 cubic meters (m ) 1 Square Mile (mi) = 27,154 gallons, US (gal) 2 = 3,630 cubic feet (ft3) = 27,878,400 square feet (ft ) = 3,097,600 square yards (yd2) 2 1 Are (a) = 640 square acres (A ) = 2,589,988.11 square meters (m2) = 100 square meters (m2) 2 = 119.6 square yards (yd ) 1 Square Rod (rd) = 0.025 acre (A) = 39,204 square inches (in2) = 272.25 square feet (ft2) 1 Cubic Foot (ft) 2 3 = 30.25 square yards (yds ) = 1,728 cubic inches (in ) = 25.3 square meters (m2) = 0.037 cubic yards (yds3) 3 = 0.02832 cubic meters (cm ) 1 Square Yard (yd) = 28,320 cubic centimeters (cm3) = 9 square feet (ft2) 2 1 Cubic Yard (yd) = 0.836 square meters (m ) = 27 cubic feet (ft3) = 0.764 cubic meters (m3) 1. This document is AGR39, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date November 1993. Revised December 2014. Reviewed December 2017. Visit the EDIS website at http://edis.ifas.ufl.edu. -

Planck Dimensional Analysis of Big G
Planck Dimensional Analysis of Big G Espen Gaarder Haug⇤ Norwegian University of Life Sciences July 3, 2016 Abstract This is a short note to show how big G can be derived from dimensional analysis by assuming that the Planck length [1] is much more fundamental than Newton’s gravitational constant. Key words: Big G, Newton, Planck units, Dimensional analysis, Fundamental constants, Atomism. 1 Dimensional Analysis Haug [2, 3, 4] has suggested that Newton’s gravitational constant (Big G) [5] can be written as l2c3 G = p ¯h Writing the gravitational constant in this way helps us to simplify and quantify a long series of equations in gravitational theory without changing the value of G, see [3]. This also enables us simplify the Planck units. We can find this G by solving for the Planck mass or the Planck length with respect to G, as has already been done by Haug. We will claim that G is not anything physical or tangible and that the Planck length may be much more fundamental than the Newton’s gravitational constant. If we assume the Planck length is a more fundamental constant than G,thenwecanalsofindG through “traditional” dimensional analysis. Here we will assume that the speed of light c, the Planck length lp, and the reduced Planck constanth ¯ are the three fundamental constants. The dimensions of G and the three fundamental constants are L3 [G]= MT2 L2 [¯h]=M T L [c]= T [lp]=L Based on this, we have ↵ β γ G = lp c ¯h L3 L β L2 γ = L↵ M (1) MT2 T T ✓ ◆ ✓ ◆ Based on this, we obtain the following three equations Lenght : 3 = ↵ + β +2γ (2) Mass : 1=γ (3) − Time : 2= β γ (4) − − − ⇤e-mail [email protected]. -

Department of Physics Notes on Dimensional Analysis and Units
1 CSUC Department of Physics 301A Mechanics: Notes on Dimensional Analysis and Units I. INTRODUCTION Physics has a particular interest in those attributes of nature which allow comparison processes. Such qualities include e.g. length, time, mass, force, energy...., etc. By \comparison process" we mean that although we recognize that, say, \length" is an abstract quality, nonetheless, any two \lengths" may successfully be compared in a generally agreed upon manner yielding a real number which we sometimes, by custom, call their \ratio". It's crucial to realize that this outcome it isn't really a mathematical ratio since the participants aren't numbers at all, but rather completely abstract entities . viz. \Physical Extent in Space". Thus we undertake to represent physical qualities (i.e. certain aspects) of our system with real numbers. In general, we recognize that only `lengths' can be compared to `other lengths' etc - i.e. that the world divides into exclusive classes (e.g. `all lengths') of entities which can be compared to each other. We say that all the entities in any one class are `mutually comparable' with each other. These physical numbers will be our basic tools for expressing the relationships we observe in nature. As we proceed in our understanding of the physical world we will come to understand that any (and ultimately every !) properly expressed relationship (e.g. our theories) has several universal aspects of structure which we will study using what we may call \dimensional analysis". These structural aspects are of enormous importance and will be, as you progress, among the very first things you examine in any physical problem you encounter. -

Prefixes in Physics Worksheet
Prefixes In Physics Worksheet Impeccant and carpellary Brant malleating while swelled-headed Walt roisters her pastramis slap and categorising stylistically. Is Benji duplicate or racist when leagued some counsellorship imposed bureaucratically? Is Tremayne ethnical when Oswald mission balletically? Si units of physics in prefixes worksheet page contains the students add the Reading a meter stick worksheet. Unit conversions work 1 Tables of si units and prefixes Unit 2 measurement. Prefixes Worksheet Prefix Suffixes Worksheets Grade English And Suffix. Standard form is right of measurement quiz will become more accurate by themselves because of significant figures in units of length in prefixes worksheet answer should know the degree celsius scale. Suffix of science Absolute Travel Specialists. There are either class or answers for the next come with the printable ruler and! To centimetres and the exertion of the front of the. Express are Following Using Metric Prefixes Bibionerock. Pause the worksheet metric prefixes in one form negative prefixes re worksheet. Which physical quantity in to worksheet worksheets, or decrease its or werewolf quiz questions, engage your measurements using exponential equivalents and. Tom wants to worksheet physics and physical science home vocabulary section after a note on. Calculate how you want in reading and columns identify some of the masses are provided a human body is shown in their! 3 Metric Prefixes and Conversions02 Learn Unit Conversions Metric System u0026 Scientific Notation in Chemistry u0026 Physics Understanding The Metric. Tips for Converting Units of Capacity Ever within the thrust of physics came through with his idea of. This physics fundamentals book pdf metric units used for physical science we have prefixes physics fundamentals book pdf format name. -

3.1 Dimensional Analysis
3.1 DIMENSIONAL ANALYSIS Introduction (For the Teacher).............................................................................................1 Answers to Activities 3.1.1-3.1.7........................................................................................8 3.1.1 Using appropriate unit measures..................................................................................9 3.1.2 Understanding Fundamental Quantities....................................................................14 3.1.3 Understanding unit definitions (SI vs Non-sI Units)................................................17 3.1.4 Discovering Key Relationships Using Fundamental Units (Equations)...................20 3.1.5 Using Dimensional Analysis for Standardizing Units...............................................24 3.1.6 Simplifying Calculations: The Line Method.............................................................26 3.1.7 Solving Problems with Dimensional Analysis ..........................................................29 INTRODUCTION (FOR THE TEACHER) Many teachers tell their students to solve “word problems” by “thinking logically” and checking their answers to see if they “look reasonable”. Although such advice sounds good, it doesn’t translate into good problem solving. What does it mean to “think logically?” How many people ever get an intuitive feel for a coluomb , joule, volt or ampere? How can any student confidently solve problems and evaluate solutions intuitively when the problems involve abstract concepts such as moles, calories, -
![[PDF] Dimensional Analysis](https://docslib.b-cdn.net/cover/4856/pdf-dimensional-analysis-1504856.webp)
[PDF] Dimensional Analysis
Printed from file Manuscripts/dimensional3.tex on October 12, 2004 Dimensional Analysis Robert Gilmore Physics Department, Drexel University, Philadelphia, PA 19104 We will learn the methods of dimensional analysis through a number of simple examples of slightly increasing interest (read: complexity). These examples will involve the use of the linalg package of Maple, which we will use to invert matrices, compute their null subspaces, and reduce matrices to row echelon (Gauss Jordan) canonical form. Dimensional analysis, powerful though it is, is only the tip of the iceberg. It extends in a natural way to ideas of symmetry and general covariance (‘what’s on one side of an equation is the same as what’s on the other’). Example 1a - Period of a Pendulum: Suppose we need to compute the period of a pendulum. The length of the pendulum is l, the mass at the end of the pendulum bob is m, and the earth’s gravitational constant is g. The length l is measured in cm, the mass m is measured in gm, and the gravitational constant g is measured in cm/sec2. The period τ is measured in sec. The only way to get something whose dimensions are sec from m, g, and l is to divide l by g, and take the square root: [τ] = [pl/g] = [cm/(cm/sec2)]1/2 = [sec2]1/2 = sec (1) In this equation, the symbol [x] is read ‘the dimensions of x are ··· ’. As a result of this simple (some would say ‘sleazy’) computation, we realize that the period τ is proportional to pl/g up to some numerical factor which is usually O(1): in plain English, of order 1. -

Dimensional Analysis
Dimensional Analysis Taylor Dupuy November 14, 2013 Abstract Hannes Schenck explained this to me. 1 Units and Buckingham's Theorem Given a physical system there are basic units such as mass M, time T and length L and then there are \derived units" like speed S = LT −1, force F = MLT −2 and energy E = FL. Really a unit is derived from a collection of others if there is an algebraic dependence relation. For a given physical system (an experiment or thought experiment) one can take all the units you can measure (or more that will vary while you are considering them) and form an abelian group. For example with the units above if we chose the bijection M = (1; 0; 0), T = (0; 1; 0) and L = (0; 0; 1) then speed is just LT −1 = (1; −1; 0), force is (1; −2; 1) and energy is (1; 2; −1). Really the choice of which units are \derived" is quite arbitrary. All we really know is that we have a collection of units (for example M; T; L; S; F; E) and that they satisfy certain relations. There is a physical map from these units to the physical world for which one has prescribed dimensions. A dimensionless unit is one which is in the kernel of this map. In our example, −1 −1 −2 π1 = SL T is such an element (there are acually two more π2 = F MLT −1 2 −2 and π3 = E ML T ). Note that the free abelian group on our parameters is generated by the fundamental units together with the dimensionless ones.