Squaliformes; Somniosidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Introduction to the Classification of Elasmobranchs
An introduction to the classification of elasmobranchs 17 Rekha J. Nair and P.U Zacharia Central Marine Fisheries Research Institute, Kochi-682 018 Introduction eyed, stomachless, deep-sea creatures that possess an upper jaw which is fused to its cranium (unlike in sharks). The term Elasmobranchs or chondrichthyans refers to the The great majority of the commercially important species of group of marine organisms with a skeleton made of cartilage. chondrichthyans are elasmobranchs. The latter are named They include sharks, skates, rays and chimaeras. These for their plated gills which communicate to the exterior by organisms are characterised by and differ from their sister 5–7 openings. In total, there are about 869+ extant species group of bony fishes in the characteristics like cartilaginous of elasmobranchs, with about 400+ of those being sharks skeleton, absence of swim bladders and presence of five and the rest skates and rays. Taxonomy is also perhaps to seven pairs of naked gill slits that are not covered by an infamously known for its constant, yet essential, revisions operculum. The chondrichthyans which are placed in Class of the relationships and identity of different organisms. Elasmobranchii are grouped into two main subdivisions Classification of elasmobranchs certainly does not evade this Holocephalii (Chimaeras or ratfishes and elephant fishes) process, and species are sometimes lumped in with other with three families and approximately 37 species inhabiting species, or renamed, or assigned to different families and deep cool waters; and the Elasmobranchii, which is a large, other taxonomic groupings. It is certain, however, that such diverse group (sharks, skates and rays) with representatives revisions will clarify our view of the taxonomy and phylogeny in all types of environments, from fresh waters to the bottom (evolutionary relationships) of elasmobranchs, leading to a of marine trenches and from polar regions to warm tropical better understanding of how these creatures evolved. -

Studies on Luminescence. on the Subocular Light-Organs of Stomiatoid Fishes
J. mar. bioi. Ass. U.K. (1960) 39,529-548 529 Printed in Great Britain STUDIES ON LUMINESCENCE. ON THE SUBOCULAR LIGHT-ORGANS OF STOMIATOID FISHES By J. A. C. NICOL The Plymouth Laboratory (Text-figs. 1-10) Many stomiatoid fishes possess a peculiar light-organ below and behind the eye, as well as other kinds of photophores. This light-organ, of diagnostic importance, is termed the subocular, postocular or cheek-organ. Stomiatoid fishes, suborder Stomiatoidei, form a suborder of the Isospondyli. Subocular organs are found in the following groups: Superfamily Stomiatoidae Stomiatidae and Chauliodontidae Superfamily Astronesthoidae (= Gymnophotodermi) Astronesthidae, Melanostomiatidae and Idiacanthidae. Over the course of the past 8 years I have collected specimens of species belonging to each of the above families, and this material has made possible a comparative study of the sub ocular organs of the Stomiatoidei. MATERIALS AND METHODS Specimens of stomiatoid fishes were obtained from deep-sea catches of the R.R. S. 'Discovery II' and R.V. ' Sarsia '. I am indebted to the Director of the National Institute of Oceanography for the former material. The following species were examined. Stomiatidae Stomias brevibarbatus Ege, 1918. One specimen, 'Discovery' station No. 3354. S. ferox Reinhardt (Zugmayer, 19II; Ege, 1918). Four specimens, 'Sarsia' stations No. 7/1957, No. II (Kon & Fisher)jI959, No. 15/1959. Chauliodontidae Chauliodus sloani Schneider (Regan & Trewavas, 1929). One specimen, 'Discovery' station No. 3051. Astronesthidae Astronesthf-s elucens Brauer (Parr, 1927). Two specimens, 'Sarsia' stations Nos. 23/1957, 14/1959. 33 JOURN. MAR. BIOL. ASSOC. VOL. 39, J960 530 J. A. C. NICOL Astronesthes richardsoni Poey (Parr, 1927). -

Squaliform Shark Teeth of the Genus Centroselachus from the Miocene of Japan
Jour. Geol. Soc. Japan, Vol. 114, No. 10, p. 536-539, October 2008 Squaliform shark teeth of the genus Centroselachus from the Miocene of Japan Hideshi Suzuki *† Received April 21, 2008. Accepted September 24, 2008. * Graduate school of Natural Science and Technology, Division of Environmental Science and Engineering, Kanazawa Uni- versity, Kakuma, Kanazawa 920-1192, Japan † Tateshina Senior High School, 3652, Ashida, Tateshina Town, Kitasaku Gun, Nagano 384-2305 Japan. Abstract: Newly found fossil shark teeth of a sleeper shark are described. This is probably an undescribed species of the genus Centroselachus belonging to the Family Somniosidae. These fossil teeth were discovered from the Middle Miocene Iseyama Formation(Northern Fossa Magna Region), Ueda City, Nagano Prefecture, central Japan. These teeth indicate the shape of a part of a symphysial tooth row, which belong to a left side of Fig. 1. Map showing the fossil locality of Centroselachus sp. ※: parasymphysial teeth and a left first anterior tooth. 138 °18 ′00.11 ″E, 36 °25 ′12.05 ″N, using the topographical map Judging from the main characters of a parasymphysial of“Sanada”, scale 1: 25,000, published by Geographical Survey tooth, such as distal and mesial blades presented both Institute. joining in a notch, it is considered that the tooth differs from teeth of the genus Centroscymnus. This fossil is identified as the genus Centroselachus sp.. This paper constitutes the first fossil record of the genus Cen- troselachus from the Miocene of Japan. Key words: Centroselachus, Somniosidae, Middle Miocene, Iseyama Formation, Northern Fossa Magna Region, Nagano Prefecture, symphysial tooth row. Introduction The purpose of this study is to report newly found fossil shark teeth of the sleeper shark of the genus Centroselachus from the Miocene of Japan. -

The First Evidence of Intrinsic Epidermal Bioluminescence Within Ray-Finned Fishes in the Linebelly Swallower Pseudoscopelus Sagamianus (Chiasmodontidae)
Received: 10 July 2019 Accepted: 22 October 2019 DOI: 10.1111/jfb.14179 BRIEF COMMUNICATION FISH The first evidence of intrinsic epidermal bioluminescence within ray-finned fishes in the linebelly swallower Pseudoscopelus sagamianus (Chiasmodontidae) Michael J. Ghedotti1,2 | W. Leo Smith3 | Matthew P. Davis4 1Department of Biology, Regis University, Denver, Colorado, USA Abstract 2Bell Museum of Natural History, University of External and histological examination of the photophores of the linebelly swallower Minnesota, St. Paul, Minnesota, USA Pseudoscopelus sagamianus reveal three epidermal layers of cells that form the light- 3Department of Ecology and Evolutionary Biology and Biodiversity Institute, University producing and light-transmitting components of the photophores. Photophores of Kansas, Lawrence, Kansas, USA among the examined photophore tracts are not significantly different in structure but 4 Department of Biological Sciences, St. Cloud the presence of mucous cells in the superficial layers of the photophore suggest con- State University, St. Cloud, Minnesota, USA tinued function of the epidermal photophore in contributing to the mucous coat. This Correspondence is the first evidence of intrinsic bioluminescence in primarily epidermal photophores Michael J. Ghedotti, Department of Biology, Regis University, 3333 Regis Boulevard, reported in ray-finned fishes. Denver, CO, 80221-1099, USA. Email: [email protected] KEYWORDS Funding information bioluminescence, deep-sea, histology, integument, photophores, Pseudoscopelus sagamianus, The work primarily was supported by funding Scombriformes from a Regis URSC Grant to M.J.G., a University of Kansas GRF allocation (#2105077) to W.L.S. and National Science Foundation grants (DEB 1258141 and DEB 1543654) to M.P.D. and W.L.S. provided monetary support. -

Morphology and Significance of the Luminous Organs in Alepocephaloid Fishes
Uiblein, F., Ott, J., Stachowitsch,©Akademie d. Wissenschaften M. (Eds), Wien; 1996: download Deep-sea unter www.biologiezentrum.at and extreme shallow-water habitats: affinities and adaptations. - Biosystematics and Ecology Series 11:151-163. Morphology and significance of the luminous organs in alepocephaloid fishes Y. I. SAZONOV Abstract: Alepocephaloid fishes, or slickheads (two families, Alepocephalidae and Platytroctidae), are deep-sea fishes distributed in all three major oceans at depths of ca. 100-5000 m, usually between ca. 500 and 3000 m. Among about 150 known species, 13 alepocephalid and all (ca. 40) platytroctid species have diverse light organs: 1) postcleithral luminous gland (all platytroctids); it releases a luminescent secredon which presumably startles or blinds predators and allows the fish to escape; 2) relatively large, regulär photophores on the head and ventral parts of the body in the alepocephalid Microphotolepis and in 6 (of 14) platytroctid genera. These organs may serve for countershading and possibly for giving signals to other individuals of the same species; 3) small "simple" or "secondary" photophores covering the whole body and fins in 5 alepocephalid genera, and a few such structures in 1 platytroctid; these organs may be used for Camouflage in the glow of spontaneous bioluminescence; 4) the mental light organ in 2 alepocephalid genera from the abyssal zone (Bathyprion and Mirognathus) may be used as a Iure to attract prey. Introduction Alepocephaloid fishes, or slickheads, comprise two families of isospondyl- ous fishes (Platytroctidae and Alepocephalidae). The group is one of the most diverse among oceanic bathypelagic fishes (about 35 genera with 150 species), and these fishes play a significant role in the communities of meso- and bathypelagic animals. -

“Trophic Strategies and Basic Morphology of the Rare Demersal Kitefin Shark Dalatias Licha in the North- Western Mediterranean Sea”
“Trophic strategies and basic morphology of the rare demersal kitefin shark Dalatias licha in the north- western Mediterranean Sea” Mª Lourdes López Calero Master in Marine Sciences: Oceanography and Marine Environment Management Directores: Tutor: Dra. Marta Coll Montón y Dr. Joan Dr. Joan Baptista Company Claret Navarro Bernabé Departamento de Recursos Marinos Departamento de Recursos Marinos Renovables Renovables Instituto de Ciencias del Mar - CSIC Instituto de Ciencias del Mar - CSIC September 2013 Abstract At the present time unravelling the complexity of marine food webs has become a valuable tool to progress on the knowledge on how marine ecosystems are structured and how the function. Recently, to the study of species trophic ecology, has been applied a combination of two complementary methodologies, stomach content and stable isotope analyses With the present study I aimed at examining the trophic ecology and other biological traits of the kitefin shark Dalatias licha in the north-western Mediterranean Sea. A demersal shark which has been assessed as near threaten globally and data deficient in the Mediterranean Sea within the IUCN framework. In this study I provide morphological measures and also new information on its diet composition, trophic ecology and trophic level of this organism by means of stomach content analysis, providing dietary quantitative indexes (%F, %N and %IRI), and stable isotopes analysis by measuring δ15N and δ13C isotopic values and applying Bayesian isotopic mixing models. Also potential differences in dietary habits and basic morphology between sexes and different study areas were examined. Results show alimentary preference for small demersal sharks, followed by bony fishes, which could be a symptom of a dietary shift from fishes that were the main prey 30 years ago. -
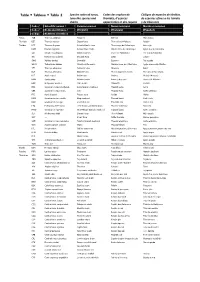
Table Tableau Tabla 2
Table Tableau Tabla 2 Species codes of tunas, Codes des espèces de Códigos de especies de túnidos, tuna‐like species and thonidés, d’espèces de especies afines a los túnidos sharks apparentées et des requins y de tiburones Code / Scientific names / Common names Noms communs Nombres comunes Code / Noms sientifiques / (English) (Français) (Español) Código Nombres científicos Tunas ALB Thunnus alalunga Albacore Germon Atún blanco Thonidés BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo) Patudo Túnidos BFT Thunnus thynnus Atlantic bluefin tuna Thon rouge de l’atlantique Atún rojo BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico SKJ Katsuwonus pelamis Skipjack tuna Listao Listado SWO Xiphias gladius Swordfish Espadon Pez espada WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico YFT Thunnus albacares Yellowfin tuna Albacore Rabil BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras BLT Auxis rochei Bullet tuna Bonitou Melva(=Melvera) BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del Atlántico BOP Orcynopsis unicolor Plain bonito Palomette Tasarte BRS Scomberomorus brasiliensis Serra Spanish mackerel Thazard serra Serra CER Scomberomorus regalis Cero Thazard franc Carite chinigua FRI Auxis thazard Frigate tuna Auxide Melva KGM Scomberomorus cavalla King mackerel Thazard barré Carite lucio KGX Scomberomorus spp -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught. -

Occurrence of Mature Female of Etmopterus Spinax (Chondrichthyes: Etmopteridae) in the Syrian Coast (Eastern Mediterranean)
ISSN: 2687-8089 DOI: 10.33552/AOMB.2018.01.000504 Advances in Oceanography & Marine Biology Case Report Copyright © All rights are reserved by Adib Saad Occurrence of Mature Female of Etmopterus Spinax (Chondrichthyes: Etmopteridae) in the Syrian Coast (Eastern Mediterranean) Adib Saad* and Hasan Alkusairy Tishreen University, Lattakia, Syria Received Date: August 25, 2018 *Corresponding author: Adib Saad, Tishreen University, Lattakia, Syria. Published Date: Septembert 21, 2018 Abstract Velvet Belly Lantern shark, Etmopterus spinax (Linnaeus, 1758) is recorded for second time in the Syrian coast. A mature female of E. spinax measured 354mm total length, and weighed 208.3g, caught at depth about 300m. the specimen was in stage 3, had two ovaries; contained 9 oocytes measured between 10-5mm in diameter. The width of left and right oviducal gland measured; 8mm and 7mm, respectively. It had left and right functional uterus, white color measured; 77mm and 75mm in length; 11mm and 10 mm in width, respectively. Keywords: Velvet belly lantern shark; Second record; Maturing, Syrian coast Introduction following the scales for viviparous Elasmobranchs proposed by The velvet belly Etmopterus spinax (Linnaeus, 1758) is a small- Anonymous [12]. sized deep-water squaliform. Although E. spinax is known to be more commonly caught in the western Mediterranean Basin [1,2]; mainly off the Tunisian and Sicilian coasts [3,4], the species is reported in both Mediterranean Basins in waters ranging between 150-200 m and 400 m, and probably deeper [5]. It has been recorded at depths as low as 2,200 m in the Ionian Sea [6]. The species is reported in the Aegean Sea [7], in Turkish waters [8], off the Egyptian coast [9], and in the Levant Basin [10,11]. -

Coelho Phd Lantern S
UNIVERSIDADEdo ALGARVE FaculdadedeCiênciasdoMaredo Ambiente Biology,populationdynamics,managementandconservation ofdeepwaterlanternsharks,Etmopterusspinax and Etmopteruspusillus (Chondrichthyes:Etmopteridae)insouthernPortugal(northeastAtlantic). (DoutoramentoemCiênciaseTecnologiasdasPescas,especialidadedeBiologiaPesqueira) (ThesisforthedegreeinDoctorofPhilosophyinFisheriesSciencesandTechnologies,specialtyinFisheriesBiology) RUIPEDROANDRADECOELHO Faro (2007) UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS DO MAR E DO AMBIENTE Biology, population dynamics, management and conservation of deep water lantern sharks, Etmopterus spinax and Etmopterus pusillus (Chondrichthyes: Etmopteridae) in southern Portugal (northeast Atlantic). (Doutoramento em Ciências e Tecnologias das Pescas, especialidade de Biologia Pesqueira) (Thesis for the degree in Doctor of Philosophy in Fisheries Sciences and Technologies, specialty in Fisheries Biology) RUI PEDRO ANDRADE COELHO Orientador / Supervisor: Prof. Doutor Karim Erzini Júri / Jury: - Prof. Doutor José Pedro Andrade, Professor Catedrático da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Karim Erzini, Professor Associado com Agregação da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Leonel Paulo Sul de Serrano Gordo, Professor Auxiliar com Agregação da Faculdade de Ciências, Universidade de Lisboa; - Prof. Doutor Manuel Seixas Afonso Dias, Professor Auxiliar da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; -

Integrating Multiple Chemical Tracers to Elucidate the Diet and Habitat of Cookiecutter Sharks Aaron B
www.nature.com/scientificreports OPEN Integrating multiple chemical tracers to elucidate the diet and habitat of Cookiecutter Sharks Aaron B. Carlisle1*, Elizabeth Andruszkiewicz Allan2,9, Sora L. Kim3, Lauren Meyer4,5, Jesse Port6, Stephen Scherrer7 & John O’Sullivan8 The Cookiecutter shark (Isistius brasiliensis) is an ectoparasitic, mesopelagic shark that is known for removing plugs of tissue from larger prey, including teleosts, chondrichthyans, cephalopods, and marine mammals. Although this species is widely distributed throughout the world’s tropical and subtropical oceanic waters, like many deep-water species, it remains very poorly understood due to its mesopelagic distribution. We used a suite of biochemical tracers, including stable isotope analysis (SIA), fatty acid analysis (FAA), and environmental DNA (eDNA), to investigate the trophic ecology of this species in the Central Pacifc around Hawaii. We found that large epipelagic prey constituted a relatively minor part of the overall diet. Surprisingly, small micronektonic and forage species (meso- and epipelagic) are the most important prey group for Cookiecutter sharks across the studied size range (17–43 cm total length), with larger mesopelagic species or species that exhibit diel vertical migration also being important prey. These results were consistent across all the tracer techniques employed. Our results indicate that Cookiecutter sharks play a unique role in pelagic food webs, feeding on prey ranging from the largest apex predators to small, low trophic level species, in particular those that overlap with the depth distribution of the sharks throughout the diel cycle. We also found evidence of a potential shift in diet and/or habitat with size and season. -

Age and Growth of Spiny Dogfish Squalus Acanthias (Squalidae: Chondrichthyes) in the North Aegean Sea
Pakistan J. Zool., vol. 48(4), pp. 1185-1191, 2016. Age and Growth of Spiny Dogfish Squalus acanthias (Squalidae: Chondrichthyes) in the North Aegean Sea Cahide Cigdem Yıgın* and Ali Ismen Article Information Marine Science and Technology Faculty, Çanakkale Onsekiz Mart University, Received 7 January 2015 17100 Çanakkale/Turkey Revised Accepted 10 January 2015 Available online 1 July 2016 A B S T R A C T Authors’ Contribution Male and female spiny dogfish Squalus acanthias were collected in the North Aegean Sea from Both the authors conceived and Saros Bay between February 2005 and September 2008. Squalus acanthias ranged from 17.1 to designed the study and wrote the 121.6 cm in total length. Age was estimated using the second dorsal spine of 345 spiny dogfish. The article. CCY collected and analyzed coefficent of variation estimated value was 11.1%. Both male and female spiny dogfish readhed 7 the data. years of age. Estimates of the von Bertalanffy growth parameters suggest that females attain a larger Key words -1 asymptotic TL (L∞=101.21 cm) than males do (L∞=72.85 cm) and grow more slowly (K=0.15 y and Squalus acanthias, spiny dogfish, age, -1 0.27 y , respectively). A relationship was determined between the age of spiny dogfish and spine growth, dorsal spine, North Aegean size: their spine length and spine base length (rs=0.625), and their spine base width (rs=0.611). Sea. INTRODUCTION unique among elasmobranchs as they usually have well- calcified dorsal spines that can be used for age estimation (Kaganovskaia, 1933; Templeman, 1944; Probatov, 1957; The spiny dogfish (Squalus acanthias) is a small Holden and Meadows, 1962; Ketchen, 1975; Beamish demersal shark of temperate continental shelf areas and McFarlane, 1985; Rice et al., 2009).