The Propagation of Sunlight And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seasonal and Spatial Variations in the Attenuation of Light in the North Atlantic Ocean
JEFFREY H. SMART SEASONAL AND SPATIAL VARIATIONS IN THE ATTENUATION OF LIGHT IN THE NORTH ATLANTIC OCEAN This article examines seasonal and spatial vanatlOns in the average depth profiles of the diffuse attenuation coefficient, Kd, for visible light propagating downward from the ocean surface at wavelengths of 490 and 532 nm. Profiles of the depth of the nth attenuation length are also shown. Spring, summer, and fall data from the northwestern and north central Atlantic Ocean and winter data from the northeastern Atlantic Ocean are studied. Variations in the depth-integrated Kd profiles are important because they are related to the depth penetration achievable by an airborne active optical antisubmarine warfare system. INTRODUCTION Numerous investigators have tried to characterize the the geographic areas and seasons indicated in Table 1 and diffuse attenuation coefficient (hereafter denoted as Kd) Figure 1. Despite the incomplete spatial and temporal for various ocean areas and for the different seasons. Platt coverage across the North Atlantic Ocean, this article and Sathyendranath 1 generated numerical fits to averaged should serve as a useful resource to estimate the optical profiles from coastal and open-ocean regions, and they characteristics in the areas studied. divided the North Atlantic into provinces that extended The purpose of this article is to characterize the sea from North America to Europe and over large ranges sonal and spatial variations in diffuse attenuation at 490 in latitude (e.g. , from 10.0° to 37.00 N and from 37.0° to and 532 nm. Because Kd frequently changes with depth, 50.00 N). In addition, numerous papers have attempted profiles of depth versus number of attenuation lengths are to model chlorophyll concentrations (which are closely required to characterize the attenuation of light as it correlated with Kd ) using data on available sunlight as a propagates from the surface downward into the water function of latitude and season, nutrient concentrations, column. -

Natural and Enhanced Attenuation of Soil and Ground Water At
LMS/MON/S04243 Office of Legacy Management Natural and Enhanced Attenuation of Soil and Groundwater at Monument Valley, Arizona, and Shiprock, New Mexico, DOE Legacy Waste Sites 2007 Pilot Study Status Report June 2008 U.S. Department OfficeOffice ofof LegacyLegacy ManagementManagement of Energy Work Performed Under DOE Contract No. DE–AM01–07LM00060 for the U.S. Department of Energy Office of Legacy Management. Approved for public release; distribution is unlimited. This page intentionally left blank LMS/MON/S04243 Natural and Enhanced Attenuation of Soil and Groundwater at Monument Valley, Arizona, and Shiprock, New Mexico, DOE Legacy Waste Sites 2007 Pilot Study Status Report June 2008 This page intentionally left blank Contents Acronyms and Abbreviations ....................................................................................................... vii Executive Summary....................................................................................................................... ix 1.0 Introduction......................................................................................................................1–1 2.0 Monument Valley Pilot Studies.......................................................................................2–1 2.1 Source Containment and Removal..........................................................................2–1 2.1.1 Phreatophyte Growth and Total Nitrogen...................................................2–1 2.1.2 Causes and Recourses for Stunted Plant Growth........................................2–5 -

Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 Kev to 100 Gev*
1 of Stanaaros National Bureau Mmin. Bids- r'' Library. Ml gEP 2 5 1969 NSRDS-NBS 29 . A111D1 ^67174 tioton Cross Sections, i NBS Attenuation Coefficients, and & TECH RTC. 1 NATL INST OF STANDARDS _nergy Absorption Coefficients From 10 keV to 100 GeV U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS T X J ". j NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics -—Radio Engineering -—Time and Frequency -—Astro- physics -—Cryogenics. -

Atmosphere Observation by the Method of LED Sun Photometry
Atmosphere Observation by the Method of LED Sun Photometry A Senior Project presented to the Faculty of the Physics Department California Polytechnic State University, San Luis Obispo In Partial Fulfillment of the Requirements of the Degree Bachelor of Science by Gregory Garza April 2013 1 Introduction The focus of this project is centered on the subject of sun photometry. The goal of the experiment was to use a simple self constructed sun photometer to observe how attenuation coefficients change over longer periods of time as well as the determination of the solar extraterrestrial constants for particular wavelengths of light. This was achieved by measuring changes in sun radiance at a particular location for a few hours a day and then use of the Langley extrapolation method on the resulting sun radiance data set. Sun photometry itself is generally involved in the practice of measuring atmospheric aerosols and water vapor. Roughly a century ago, the Smithsonian Institutes Astrophysical Observatory developed a method of measuring solar radiance using spectrometers; however, these were not usable in a simple hand-held setting. In the 1950’s Frederick Volz developed the first hand-held sun photometer, which he improved until coming to the use of silicon photodiodes to produce a photocurrent. These early stages of the development of sun photometry began with the use of silicon photodiodes in conjunction with light filters to measure particular wavelengths of sunlight. However, this method of sun photometry came with cost issues as well as unreliability resulting from degradation and wear on photodiodes. A more cost effective method was devised by amateur scientist Forrest Mims in 1989 that incorporated the use of light emitting diodes, or LEDs, that are responsive only to the light wavelength that they emit. -

Radio Astronomy
Edition of 2013 HANDBOOK ON RADIO ASTRONOMY International Telecommunication Union Sales and Marketing Division Place des Nations *38650* CH-1211 Geneva 20 Switzerland Fax: +41 22 730 5194 Printed in Switzerland Tel.: +41 22 730 6141 Geneva, 2013 E-mail: [email protected] ISBN: 978-92-61-14481-4 Edition of 2013 Web: www.itu.int/publications Photo credit: ATCA David Smyth HANDBOOK ON RADIO ASTRONOMY Radiocommunication Bureau Handbook on Radio Astronomy Third Edition EDITION OF 2013 RADIOCOMMUNICATION BUREAU Cover photo: Six identical 22-m antennas make up CSIRO's Australia Telescope Compact Array, an earth-rotation synthesis telescope located at the Paul Wild Observatory. Credit: David Smyth. ITU 2013 All rights reserved. No part of this publication may be reproduced, by any means whatsoever, without the prior written permission of ITU. - iii - Introduction to the third edition by the Chairman of ITU-R Working Party 7D (Radio Astronomy) It is an honour and privilege to present the third edition of the Handbook – Radio Astronomy, and I do so with great pleasure. The Handbook is not intended as a source book on radio astronomy, but is concerned principally with those aspects of radio astronomy that are relevant to frequency coordination, that is, the management of radio spectrum usage in order to minimize interference between radiocommunication services. Radio astronomy does not involve the transmission of radiowaves in the frequency bands allocated for its operation, and cannot cause harmful interference to other services. On the other hand, the received cosmic signals are usually extremely weak, and transmissions of other services can interfere with such signals. -

Causes of Light Attenuation with Respect to Seagrasses in Upper and Lower Charlotte Harbor
Causes of Light Attenuation with Respect to Seagrasses in Upper and Lower Charlotte Harbor Final Report Submitted to: Southwest Florida Water Management District Surface Water Improvement and Management Program 7601 U.S. Highway 301 North Tampa, FL 33637 (813) 985-7481 Charlotte Harbor National Estuary Program 4980 Bayline Drive 4th Floor North Fort Myers, Florida 33917 (941) 995-1777 Submitted by: L. Kellie Dixon and Gary J. Kirkpatrick Mote Marine Laboratory 1600 Ken Thompson Parkway Sarasota, FL 34236 (941) 388-4441 September 20, 1999 Mote Marine Laboratory Technical Report No. 650. Please note that pages do not always correspond to those listed in the table of contents. This was caused when the document was converted to a PDF file. Acknowledgments Thanks are due to many, but especially Linda Franklin, Jay Leverone, Tiffany Lutterman, Ari Nissanka, Jon Perry, Joie Simoneaux, Roberta Starke, and David Tomasko. i Table of Contents Acknowledgments.............................................................................................................................i Table of Contents.............................................................................................................................ii List of Tables...................................................................................................................................iv List of Figures...................................................................................................................................v Executive Summary.........................................................................................................................ix -

Mass Attenuation Coefficients of X-Rays in Different Barite Concrete Used in Radiation Protection As Shielding Against Ionizing Radiation
X congreso Regional Latinoamericano IRPA de Protección y Seguridad Radiológica Radi opro te ce ion: Nuevos Desafíos para un Mundo en Evolución ” Buenos Aires, 12 al 17 de abril, 2015 SOCIEDAD ARGENTINA DE RADIOPROTECCIÓN MASS ATTENUATION COEFFICIENTS OF X-RAYS IN DIFFERENT BARITE CONCRETE USED IN RADIATION PROTECTION AS SHIELDING AGAINST IONIZING RADIATION Almeida Junior, Airton T.1’4’5, Nogueira, M. S.2, Santos, M. A. P3, L. L. Campos 4, Araújo, F. G. S.5, 1 Brazilian Institute for Safety and Health at Work - FUNDACENTRO, Minas Gerais, Brazil 2 Center of Development of Nuclear Technology - CDTN/CNEN, Minas Gerais, Brazil 3 Regional Center for Nuclear Science - CRCN/CNEN, Pernambuco, Brazil 4 Institute of Energy and Nuclear Research - IPEN/CNEN, São Paulo, Brazil 5 Universidade Federal de Ouro Preto - UFOP /REDEMAT, Minas Gerais, Brazil ABSTRACT The attenuation coefficient depends on the incident photon energy and the nature of the materials. In order to minimize exposure to individuals. Barite concrete has been largely used as a shielding material in installations housing gamma radiation sources as well as X-ray generating equipment. This study was conducted to evaluate the efficacy of different mixtures of barite concrete for shielding in diagnostic X-ray rooms. The mass attenuation coefficient (jn/p). The mass attenuation coefficients have been measured by employing the CdTe detector model XR-1OOT. The distance between the source and the exposed surface of all samples was measured by SSD light indicator of machine which was 350 cm. The slope of the linear plot of the intensity transmitted versus specimen thickness would yield the attenuation coefficient. -
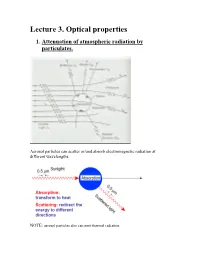
Lecture 3. Optical Properties 1
Lecture 3. Optical properties 1. Attenuation of atmospheric radiation by particulates. Aerosol particles can scatter or/and absorb electromagnetic radiation at different wavelengths. NOTE: aerosol particles also can emit thermal radiation. Scattering is a process, which conserves the total amount of energy, but the direction in which the radiation propagates may be altered. Absorption is a process that removes energy from the electromagnetic radiation field, and converts it to another form. Extinction (or attenuation) is the sum of scattering and absorption, so it represents total effect of medium on radiation passing the medium. In the atmosphere: aerosol particles can scatter and absorb solar and infrared radiation altering air temperature and the rates of photochemical reactions. Key parameters that govern the scattering and absorption of radiation by a particle: i. the wavelength of the incident radiation; ii. the size of the particles, expressed as a dimensional size parameter x: D x (where D is the particle diameter); iii. complex refractive index (or optical constant) of a particle: m = n + i k where n is the real part of the refractive index, k is the imaginary part of the refractive index. Both n and k depend on the wavelength. Important to remember: complex refractive index of a particle is defined by its chemical composition; real part , n , is responsible for scattering. imaginary part, k, is responsible for absorption. If k is equal to 0 at a given wavelength thus a particle does not absorb radiation at this wavelength. Table 3.1 Some refractive indices of atmospheric aerosol substances at = 0.5 m. -

Attenuation by Atmospheric Gases
Recommendation ITU-R P.676-11 (09/2016) Attenuation by atmospheric gases P Series Radiowave propagation ii Rec. ITU-R P.676-11 Foreword The role of the Radiocommunication Sector is to ensure the rational, equitable, efficient and economical use of the radio- frequency spectrum by all radiocommunication services, including satellite services, and carry out studies without limit of frequency range on the basis of which Recommendations are adopted. The regulatory and policy functions of the Radiocommunication Sector are performed by World and Regional Radiocommunication Conferences and Radiocommunication Assemblies supported by Study Groups. Policy on Intellectual Property Right (IPR) ITU-R policy on IPR is described in the Common Patent Policy for ITU-T/ITU-R/ISO/IEC referenced in Annex 1 of Resolution ITU-R 1. Forms to be used for the submission of patent statements and licensing declarations by patent holders are available from http://www.itu.int/ITU-R/go/patents/en where the Guidelines for Implementation of the Common Patent Policy for ITU-T/ITU-R/ISO/IEC and the ITU-R patent information database can also be found. Series of ITU-R Recommendations (Also available online at http://www.itu.int/publ/R-REC/en) Series Title BO Satellite delivery BR Recording for production, archival and play-out; film for television BS Broadcasting service (sound) BT Broadcasting service (television) F Fixed service M Mobile, radiodetermination, amateur and related satellite services P Radiowave propagation RA Radio astronomy RS Remote sensing systems S Fixed-satellite service SA Space applications and meteorology SF Frequency sharing and coordination between fixed-satellite and fixed service systems SM Spectrum management SNG Satellite news gathering TF Time signals and frequency standards emissions V Vocabulary and related subjects Note: This ITU-R Recommendation was approved in English under the procedure detailed in Resolution ITU-R 1. -

Electromagnetic Attenuation Properties of Clay and Gravel Soils Were Measured As a Function of Moisture Content and Frequency
NBSIR 74-381 ELECTROMAGNETIC ATTENUATION PROPERTIES OF CLAY AND GRAVEL SOILS Doyle A. Ellerbruch Electromagnetics Division Institute for Basic Standards National Bureau of Standards Boulder, Colorado 80302 August 1974 NBSIR 74-381 ELECTROMAGNETIC ATTENUATION PROPERTIES OF CLAY AND GRAVEL SOILS Doyle A. Ellerbruch Electromagnetics Division Institute for Basic Standards National Bureau of Standards Boulder, Colorado 80302 August 1974 u s DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS Richard W Roberts Director . FOREWORD In response to a request from the Air Force Weapons Laboratory (AFWL) the National Bureau of Standards undertook a project to investigate the microwave penetrability of selected pavement materials. The overall objective of the AFWL was to establish the feasibility of using active microwave techniques to differentiate layers in a pavement system and to accurately measure the thickness of each layer for a variety of soil types and moisture conditions. Layer thickness measurements are needed to comple- ment a field operational method currently being developed by AFWL to nondestructively evaluate the condition and load-carrying capacity of airfield pavements This initial effort consisted of investigating the relationship between microwave penetrability in clay and gravel soils as a function of moisture content and microwave frequency. ii CONTENTS Page 1. INTRODUCTION 1 2. THEORETICAL MODEL 2 3. PREPARATION OF SOIL SAMPLES 7 3.1 Gravel 7 3.2 Clay 10 4. EXPERIMENTAL RESULTS AND DISCUSSION 14 5. CONCLUSIONS 17 6. ACKNOWLEDGMENTS 19 7. REFERENCES 19 ILLUSTRATIONS Figure Page 1. Plane waves normally incident on plane dielectric boundary 4 2. Reflection coefficient relationship 6 3. Grain size distribution of gravel samples 8 4. -
Bone Mineral Density (BMD) and Tissue Mineral Density (TMD) Calibration and Measurement by Micro-CT Using Bruker-Microct CT-Analyser
HU and BMD calibration in Bruker-MicroCT CT-analyser Method note Bone mineral density (BMD) and tissue mineral density (TMD) calibration and measurement by micro-CT using Bruker-MicroCT CT-Analyser Page 1 of 30 HU and BMD calibration in Bruker-MicroCT CT-analyser 1. INTRODUCTION 1.1. What is “density” measured by x-ray micro-CT? A micro-CT 3D image of an object is basically a 3D map of local x-ray absorption in the object. The reconstruction from multiple angular views provides information on how much x-ray absorption is happening within each cubic voxel element of the scanned volume. It is important to understand what is meant by “density” in a microCT image. The reconstructed grey-scale intensity of each image voxel does not relate directly to mass density alone, thus do not expect it to correspond with measurements of weight per volume, e.g. g.cm-3. The primary entity that is measured is x- ray absorption, defined as the attenuation coefficient in units of 1/distance, specifically, mm-1. This is determined both by mass density and elemental composition of the material. However, when we know that the x-ray absorption of a material is dominated by one specific material, then we can relate the measured x- ray attenuation coefficient (AC) to the mass density of that material. The calibration of bone mineral density (BMD) is an example of this. We assume that the x-ray attenuation within mineralised tissues such as bone, dentine and enamel is dominated by, and can be approximated as, the x-ray attenuation of the mineral compound calcium hydroxyapatite (CaHA) which has the formula Ca5(PO4)3(OH). -

Evaluation of Natural Attenuation of Soil Contaminants Soil Treatability Studies Area IV Santa Susana Field Laboratory Ventura County, California
Evaluation of Natural Attenuation of Soil Contaminants Soil Treatability Studies Area IV Santa Susana Field Laboratory Ventura County, California Soil Partitioning Mercury Contamination Bioremediation Phytoremediation Natural Attenuation Study Plan Soil Treatability Studies Area IV Santa Susana Field Laboratory Ventura County, California Evaluation of Natural Attenuation of Soil Contaminants Prepared for: Department of Energy Energy Technology and Engineering Center 4100 Guardian Street Simi Valley, California 93063 Prepared by: CDM Federal Programs Corporation (CDM Smith) 555 17th Street, Suite 1200 Denver, Colorado 80202 Prepared under: US Department of Energy EM Consolidated Business Center Contract DE‐EM0001128 CDM Smith Task Order DE‐DT0003515 November 2013 This page intentionally left blank. Study Plan Soil Treatability Studies Area IV Santa Susana Field Laboratory Ventura County, California Evaluation of Natural Attenuation of Soil Contaminants Contract DE‐EM0001128 CDM Smith Task Order DE‐DT0003515 Prepared by: November 1, 2013 Keegan L. Roberts, Ph.D. Date CDM Smith Environmental Engineer Exp Oct 2014 Approved by: November 1, 2013 Michael Hoffman, P.G. Date CDM Smith Geologist Approved by: November 1, 2013 John Wondolleck Date CDM Smith Project Manager This page intentionally left blank. Table of Contents Section 1 Introduction ................................................................................................................................................ 1-1 1.1 Purpose of Study ........................................................................................................................................................