Vasopressor & Inotrope Infusions Via Peripheral Intravenous Administration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for NON - CRITICAL CARE Staff Common Vasoactive Drugs These Drugs Are Used to Maintain Cardiovascular Stability
Guidelines for NON - CRITICAL CARE staff Common vasoactive drugs These drugs are used to maintain cardiovascular stability. The full guide to administration can be found on BSUH infonet > intensive care unit > clinical guidelines > inotropes These drugs MUST be given via a central venous catheter. Be given via a ‘dedicated’ line (several vasoactives can run together in one lumen of a CVC) with a 2, 3 or 4 lumen connector. given via an ICU specific syringe driver – these allow you to change the rate without pausing the infusion. All ICU pumps have ICU or HDU spray-painted on them. Be ‘double pumped’ ie: when changing syringe, the old and new infusions run concurrently to prevent loss of infusion during change UNLESS ‘RAPID CHANGE- OVER’ TECHNIQUE IS USED, DUE TO LACK OF AVAILABLE PUMPS These drugs MUST NOT be paused, stopped or disconnected suddenly – they have a short half-life and pausing/stopping/disconnection may cause rapid CVS deterioration or arrest UNLESS ‘RAPID CHANGE-OVER’ TECHNIQUE IS USED, DUE TO LACK OF AVAILABLE PUMPS be given as a bolus, or bolused via the pump – this could cause rapid CVS instability run with ANY drug other than a vasoactive drug COMMON VASOACTIVES NORADRENALINE – vasopressor: causes vasoconstriction and used to improve BP USES: sepsis, septic shock, severe hypotension not resolved with fluid ADRENALINE – inotrope: increases contractility, raises BP and HR USES: sepsis, septic shock, severe bradycardia DOBUTAMINE – intrope and vasodilator: increases contractility and reduces cardiac work USES: -

Nitric Oxide, the Biological Mediator of the Decade: Fact Or Fiction?
Eur Respir J 1997; 10: 699–707 Copyright ERS Journals Ltd 1997 DOI: 10.1183/09031936.97.10030699 European Respiratory Journal Printed in UK - all rights reserved ISSN 0903 - 1936 SERIES 'CLINICAL PHYSIOLOGY IN RESPIRATORY INTENSIVE CARE' Edited by A. Rossi and C. Roussos Number 14 in this Series Nitric oxide, the biological mediator of the decade: fact or fiction? S. Singh, T.W. Evans Nitric oxide, the biological mediator of the decade: fact or fiction? S. Singh, T.W. Evans. Unit of Critical Care, National Heart & ERS Journals Ltd 1997. Lung Institute, Royal Brompton Hospital, ABSTRACT: Nitric oxide (NO), an atmospheric gas and free radical, is also an London, UK. important biological mediator in animals and humans. Its enzymatic synthesis by constitutive (c) and inducible (i) isoforms of NO synthase (NOS) and its reactions Correspondence: T.W. Evans with other biological molecules such as reactive oxygen species are well charac- Royal Brompton Hospital Sydney Street terized. NO modulates pulmonary and systemic vascular tone through its vasodila- London SW3 6NP tor property. It has antithrombotic functions and mediates some consequences of UK the innate and acute inflammatory responses; cytokines and bacterial toxins induce widespread expression of iNOS associated with microvascular and haemodynam- Keywords: Acute respiratory distress syn- ic changes in sepsis. drome Within the lungs, a diminution of NO production is implicated in pathological hypoxic pulmonary vasoconstriction states associated with pulmonary hypertension, such as acute respiratory distress nitric oxide syndrome: inhaled NO is a selective pulmonary vasodilator and can improve ven- nitric oxide synthase tilation-perfusion mismatch. However, it may have deleterious effects through mod- pulmonary hypertension ulating hypoxic pulmonary vasoconstriction. -

Wednesday, July 10, 2019 4:00Pm
Wednesday, July 10, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – July 10, 2019 DATE: July 3, 2019 NOTE: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the July meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/SoonerPsych Program Update – Appendix B Action Item – Vote to Prior Authorize Jornay PM™ [Methylphenidate Extended-Release (ER) Capsule], Evekeo ODT™ [Amphetamine Orally Disintegrating Tablet (ODT)], Adhansia XR™ (Methylphenidate ER Capsule), and Sunosi™ (Solriamfetol Tablet) – Appendix C Action Item – Vote to Prior Authorize Balversa™ (Erdafitinib) – Appendix D Action Item – Vote to Prior Authorize Annovera™ (Segesterone Acetate/Ethinyl Estradiol Vaginal System), Bijuva™ (Estradiol/Progesterone Capsule), Cequa™ (Cyclosporine 0.09% Ophthalmic Solution), Corlanor® (Ivabradine Oral Solution), Crotan™ (Crotamiton 10% Lotion), Gloperba® (Colchicine Oral Solution), Glycate® (Glycopyrrolate Tablet), Khapzory™ (Levoleucovorin Injection), Qmiiz™ ODT [Meloxicam Orally Disintegrating Tablet (ODT)], Seconal Sodium™ (Secobarbital -

Medicare National Coverage Determinations Manual, Part 1
Medicare National Coverage Determinations Manual Chapter 1, Part 1 (Sections 10 – 80.12) Coverage Determinations Table of Contents (Rev. 10838, 06-08-21) Transmittals for Chapter 1, Part 1 Foreword - Purpose for National Coverage Determinations (NCD) Manual 10 - Anesthesia and Pain Management 10.1 - Use of Visual Tests Prior to and General Anesthesia During Cataract Surgery 10.2 - Transcutaneous Electrical Nerve Stimulation (TENS) for Acute Post- Operative Pain 10.3 - Inpatient Hospital Pain Rehabilitation Programs 10.4 - Outpatient Hospital Pain Rehabilitation Programs 10.5 - Autogenous Epidural Blood Graft 10.6 - Anesthesia in Cardiac Pacemaker Surgery 20 - Cardiovascular System 20.1 - Vertebral Artery Surgery 20.2 - Extracranial - Intracranial (EC-IC) Arterial Bypass Surgery 20.3 - Thoracic Duct Drainage (TDD) in Renal Transplants 20.4 – Implantable Cardioverter Defibrillators (ICDs) 20.5 - Extracorporeal Immunoadsorption (ECI) Using Protein A Columns 20.6 - Transmyocardial Revascularization (TMR) 20.7 - Percutaneous Transluminal Angioplasty (PTA) (Various Effective Dates Below) 20.8 - Cardiac Pacemakers (Various Effective Dates Below) 20.8.1 - Cardiac Pacemaker Evaluation Services 20.8.1.1 - Transtelephonic Monitoring of Cardiac Pacemakers 20.8.2 - Self-Contained Pacemaker Monitors 20.8.3 – Single Chamber and Dual Chamber Permanent Cardiac Pacemakers 20.8.4 Leadless Pacemakers 20.9 - Artificial Hearts And Related Devices – (Various Effective Dates Below) 20.9.1 - Ventricular Assist Devices (Various Effective Dates Below) 20.10 - Cardiac -

FACT SHEET for HEALTHCARE PROVIDERS Coronavirus Emergency Use of the Coviage System During the COVID-19 Pandemic Disease 2019 September 24, 2020 (COVID-19)
FACT SHEET FOR HEALTHCARE PROVIDERS Coronavirus Emergency Use of the COViage System During the COVID-19 Pandemic Disease 2019 September 24, 2020 (COVID-19) This Fact Sheet informs you of the significant known and What do I need to know about COVID-19 treatment? potential risks and benefits of the emergency use of the Current information on COVID-19 infection, including COViage System (or “COViage”) for use by healthcare case definitions and information about clinical signs and providers (HCP) in the hospital setting for adult patients symptoms and/or epidemiological criteria, is available on (18 years of age or older who are admitted to the the CDC website listed at the end of this fact sheet. hospital) with confirmed COVID-19 (based on a positive . PCR test result) for the computation of proprietary patient status indices referred to as Respiratory What is the COViage System? Decompensation Status and Hemodynamic Instability The COViage System is a non-interventional software Status as an adjunct to patient monitoring during the program that uses vital signs and patient demographic COVID-19 outbreak. The COViage indices provide HCP data from EMR systems. It uses models derived from with predictive screening information as a diagnostic aid machine learning on patient data from EMRs to calculate to assist with the early identification of COVID-19 the likelihood of the occurrence of certain clinically patients who are likely to be diagnosed with significant events, specifically, hemodynamic instability hemodynamic instability or respiratory decompensation, or respiratory decompensation. It then notifies HCP of which are common complications associated with patients who, according to the algorithm, are expected to COVID-19. -

Caring for Patients Receiving Vasopressors and Inotropes in the ICU
Caring for patients receiving vasopressors and inotropes in the ICU Vigilant monitoring will maximize outcomes. By Sonya M. Grigsby, DNP, APRN, AGACNP-BC CNE 1.5 contact hours LEARNING O BJECTIVES 1. Identify receptors related to cardiovascular physiology. 2. Differentiate the effects of vasopressors and inotropes. 3. Discuss nursing care of patients receiving vasopressors and inotropes in the ICU. The authors and planners of this CNE activity have disclosed no relevant fi- nancial relationships with any commercial companies pertaining to this ac- tivity. See the last page of the article to learn how to earn CNE credit. Expiration: 2/1/24 SHOCK requires early recognition and quick peutic effect) these medications using the action to prevent organ failure. (See Types of lowest possible dose to avoid adverse effects. shock.) After initial I.V. fluid resuscitation, pharmacologic agents—such as vasopressors Cardiovascular physiology and inotropes—are used in critical care set- Shock disrupts cardiovascular physiology, par- tings as supportive therapies to improve my- ticularly blood pressure, so understanding ocardial contractility, heart rate, and vascular normal function is important. Short-term resistance in patients with low cardiac output. blood pressure (BP) regulation is controlled When critical care nurses understand shock by the autonomic nervous system (ANS) via pathophysiology and hemodynamic monitor- baroreceptors in the aortic arch and carotid si- ing, they can effectively and safely titrate (in- nus. The ANS regulates the heart, secretory crease or decrease an infusion rate for thera- glands, and smooth muscles via activation of MyAmericanNurse.com February 2021 American Nurse Journal 5 Types of shock Shock is a decline in tissue perfusion and oxygen delivery, leading to cellular dysfunction and death. -

The Effects of Dobutamine on Cardiac Sympathetic Activity in Patients with Congestive Heart Failure Abdul Al-Hesayen, MD, Eduardo R
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Journal of the American College of Cardiology Vol. 39, No. 8, 2002 © 2002 by the American College of Cardiology Foundation ISSN 0735-1097/02/$22.00 Published by Elsevier Science Inc. PII S0735-1097(02)01783-7 The Effects of Dobutamine on Cardiac Sympathetic Activity in Patients With Congestive Heart Failure Abdul Al-Hesayen, MD, Eduardo R. Azevedo, MD, Gary E. Newton, MD, John D. Parker, MD, FACC Toronto, Canada OBJECTIVES The goal of this work was to study the effects of short-term infusion of dobutamine on efferent cardiac sympathetic activity. BACKGROUND Increased efferent cardiac sympathetic activity is associated with poor outcomes in the setting of congestive heart failure (CHF). Dobutamine is commonly used in the therapy of decompensated CHF. Dobutamine, through its effects on excitatory beta-receptors, may increase cardiac sympathetic activity. METHODS Seven patients with normal left ventricular (LV) function and 13 patients with CHF were studied. A radiotracer technique was used to measure cardiac norepinephrine spillover (CANESP) before and during an intravenous infusion of dobutamine titrated to increase the rate of rise in LV peak positive pressure (ϩdP/dt) by 40%. RESULTS Systemic arterial pulse pressure increased significantly in response to dobutamine in the normal LV function group (74 Ϯ 3mmHgto85Ϯ 3 mm Hg, p ϭ 0.005) but remained unchanged in the CHF group. Dobutamine caused a significant decrease in LV end-diastolic pressure in the CHF group (14 Ϯ 2mmHgto11Ϯ 2 mm Hg, p ϭ 0.02), an effect not observed in the normal LV group. -

Wednesday, June 12, 2019 4:00Pm
Wednesday, June 12, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – June 12, 2019 DATE: June 5, 2019 Note: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the June meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Use of Angiotensin Converting Enzyme Inhibitor (ACEI)/ Angiotensin Receptor Blocker (ARB) Therapy in Patients with Diabetes and Hypertension (HTN) Mailing Update – Appendix B Action Item – Vote to Prior Authorize Aldurazyme® (Laronidase) and Naglazyme® (Galsulfase) – Appendix C Action Item – Vote to Prior Authorize Plenvu® [Polyethylene Glycol (PEG)-3350/Sodium Ascorbate/Sodium Sulfate/Ascorbic Acid/Sodium Chloride/Potassium Chloride] – Appendix D Action Item – Vote to Prior Authorize Consensi® (Amlodipine/Celecoxib) and Kapspargo™ Sprinkle [Metoprolol Succinate Extended-Release (ER)] – Appendix E Action Item – Vote to Update the Prior Authorization Criteria For H.P. Acthar® Gel (Repository Corticotropin Injection) – Appendix F Action Item – Vote to Prior Authorize Fulphila® (Pegfilgrastim-jmdb), Nivestym™ (Filgrastim-aafi), -
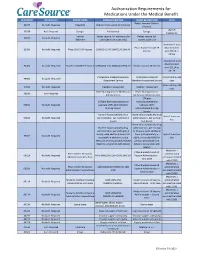
Authorization Requirements for Medications Under
Authorization Requirements for Medications Under the Medical Benefit HCPC/MOD Marketplace BRAND NAME LONG DESCRIPTION SHORT DESCRIPTION Limits Rabies Immune Globulin 90375 No Auth. Required HyperRab Rabies Immune Globulin (Human) (Human) Up to 5 90378 Auth Required Synagis Palivizumab Synagis treatments Imovax Rabies vaccine, for intramuscular Rabies vaccine, for 90675 No Auth. Required Rabavert use (Code price is per 1 mL) intramuscular use Maximum 2 units Pfizer-Biontech Covid-19 Must be billed 91300 No Auth. Required Pfizer COVID-19 Vaccine SARSCOV2 VAC 30MCG/0.3ML IM Vaccine with 0001A or 0002A Maximum 2 units Must be billed 91301 No Auth. Required Moderna COVID-19 Vaccine SARSCOV2 VAC 100MCG/0.5ML IM Moderna Covid-19 Vaccine with 0011A or 0012A Postpartum Maternal Newborn Postpartum Maternal 4 Units Within 180 99501 No Auth. Required Assessment Service Newborn Assessment Service days 4 Units Within 180 99502 No Auth. Required Newborn Assessment Newborn Assessment days Home Nursing Visit for Medication Home Nursing Visit for 99506 Auth Required Administration Medication Administration 17Alpha- 17Alpha-hydroxyprogesterone hydroxyprogesterone 99600 No Auth. Required Caproate (17P) Administration Caproate (17P) Nursing Service Administration Nursing Service Home infusion/specialty drug Home infusion/specialty drug Up to 2 hours per 99601 No Auth. Required administration, per visit (up to 2 administration, per visit (up day hours) to 2 hours) Home infusion/specialty drug Home infusion/specialty drug administration, per visit (up administration, per visit (up to 2 to 2 hours); each additional hours); each additional hour (List hour (List separately in Up to 2 hours per 99602 No Auth. Required separately in addition to code addition to code 99601 for day 99601 for primary procedure) (Use primary procedure) (Use 99602 in conjunction with 99601) 99602 in conjunction with 99601) Maximum 1 Pfizer-Biontech Covid-19 Pfizer COVID-19 Vaccine administration 0001A No Auth. -

Inorganic Nitrate As a Treatment for Acute Heart Failure
Falls et al. J Transl Med (2017) 15:172 DOI 10.1186/s12967-017-1271-z Journal of Translational Medicine PROTOCOL Open Access Inorganic nitrate as a treatment for acute heart failure: a protocol for a single center, randomized, double‑blind, placebo‑controlled pilot and feasibility study Roman Falls1,2, Michael Seman1,2, Sabine Braat2,4, Joshua Sortino1, Jason D. Allen1,3 and Christopher J. Neil1,2,3,5* Abstract Background: Acute heart failure (AHF) is a frequent reason for hospitalization worldwide and efective treatment options are limited. It is known that AHF is a condition characterized by impaired vasorelaxation, together with reduced nitric oxide (NO) bioavailability, an endogenous vasodilatory compound. Supplementation of inorganic sodium nitrate (NaNO3) is an indirect dietary source of NO, through bioconversion. It is proposed that oral sodium nitrate will favorably afect levels of circulating NO precursors (nitrate and nitrite) in AHF patients, resulting in reduced systemic vascular resistance, without signifcant hypotension. Methods and outcomes: We propose a single center, randomized, double-blind, placebo-controlled pilot trial, evaluating the feasibility of sodium nitrate as a treatment for AHF. The primary hypothesis that sodium nitrate treat- ment will result in increased systemic levels of nitric oxide pre-cursors (nitrate and nitrite) in plasma, in parallel with improved vasorelaxation, as assessed by non-invasively derived systemic vascular resistance index. Additional sur- rogate measures relevant to the known pathophysiology of AHF will be obtained in order to assess clinical efect on dyspnea and renal function. Discussion: The results of this study will provide evidence of the feasibility of this novel approach and will be of inter- est to the heart failure community. -

Postoperative Vasopressor Usage: a Prospective International Observational Study
SQUEEZE Postoperative vasopressor usage: a prospective international observational study CRF1 PATIENT IDENTIFICATION NUMBER: _____________ PRE-OPERATIVE DATE OF SURGERY: ___________________________ Month and year of birth Sex [m/f] Height [cm] Weight [kg] Clinical Frailty Scale (Rockwood): point 0 to 9. (Will be explained in final CRF) Previous medical history: Coronary Artery Disease: Y/N Cerebrovascular Disease: Y/N Peripheral vascular disease: Y/N Atrial fibrillation: Y/N Heart failure: Y/N Hypertension: Y treated and controlled, Y treated but not controlled, No Diabetes: Takes insulin/managed without insulin/None Chronic liver disease: Y/N Chronic respiratory disease: COPD/other/None Chronic immunosuppression: HIV/other/none Chronic Kidney Disease: No/Yes/Yes and receives renal replacement therapy Long-term steroid use: Y/N Recent/current treatment for cancer (including chemotherapy, radiotherapy, surgery) Regular medications ACE inhibitor: Y and took today/ Y omitted today/N Alpha blocker: Y and took today/ Y omitted today/N Angiotensin Receptor Blocker: Y and took today/ Y omitted today/N Beta blocker: Y and took today/ Y omitted today/N Calcium channel blocker: Y and took today/ Y omitted today Diuretic: Y and took today/ Y omitted today/N Regular NSAIDs: Y/N Regular PPI: Y/N Haemodynamics Measurement in the past 6 months, at least 12h prior to the operating room, at rest: Systolic, Diastolic Heart rate The reading immediately prior to induction of anaesthesia: Systolic, Diastolic Heart rate Laboratory results, most recent (if known -

What Inotrope and Why?
What Inotrope and Why? a,b, a,b Nilkant Phad, MD, FRACP *, Koert de Waal, PhD KEYWORDS Hypotension Cardiovascular compromise Inotrope Newborn KEY POINTS There are significant gaps in the knowledge about diagnostic thresholds and choices of therapeutic interventions that can improve morbidity and mortality in neonates with car- diovascular compromise. Current use of inotropes in neonates is largely based on the pathophysiology of cardiovas- cular impairment and anticipated actions of inotropes because of limited outcome data from randomized controlled trials. Research studies of alternative study design unraveling the linkage of cardiovascular impairment and use of inotropes with important clinical outcomes are needed for future progress. INTRODUCTION The primary function of the cardiovascular system is to meet oxygen and nutritional demands of organs under various physiologic and pathologic conditions.1 To achieve this, the heart contracts against vascular resistance and drives blood to the lungs for oxygenation and into the systemic circulation for organ perfusion. The force of cardiac contraction, ventricular end-diastolic blood volume, and perfusion pressure are the main determinants of cardiovascular performance through an interplay between car- diac output (CO), vascular resistance, and neuroendocrine mechanisms.2 In neonates, the physiology of blood circulation can get disrupted in many clinical conditions, resulting in impaired organ perfusion and hypoxia. Persistent circulatory compromise can lead to derangement of metabolism,