The Effects of Dobutamine on Cardiac Sympathetic Activity in Patients with Congestive Heart Failure Abdul Al-Hesayen, MD, Eduardo R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for NON - CRITICAL CARE Staff Common Vasoactive Drugs These Drugs Are Used to Maintain Cardiovascular Stability
Guidelines for NON - CRITICAL CARE staff Common vasoactive drugs These drugs are used to maintain cardiovascular stability. The full guide to administration can be found on BSUH infonet > intensive care unit > clinical guidelines > inotropes These drugs MUST be given via a central venous catheter. Be given via a ‘dedicated’ line (several vasoactives can run together in one lumen of a CVC) with a 2, 3 or 4 lumen connector. given via an ICU specific syringe driver – these allow you to change the rate without pausing the infusion. All ICU pumps have ICU or HDU spray-painted on them. Be ‘double pumped’ ie: when changing syringe, the old and new infusions run concurrently to prevent loss of infusion during change UNLESS ‘RAPID CHANGE- OVER’ TECHNIQUE IS USED, DUE TO LACK OF AVAILABLE PUMPS These drugs MUST NOT be paused, stopped or disconnected suddenly – they have a short half-life and pausing/stopping/disconnection may cause rapid CVS deterioration or arrest UNLESS ‘RAPID CHANGE-OVER’ TECHNIQUE IS USED, DUE TO LACK OF AVAILABLE PUMPS be given as a bolus, or bolused via the pump – this could cause rapid CVS instability run with ANY drug other than a vasoactive drug COMMON VASOACTIVES NORADRENALINE – vasopressor: causes vasoconstriction and used to improve BP USES: sepsis, septic shock, severe hypotension not resolved with fluid ADRENALINE – inotrope: increases contractility, raises BP and HR USES: sepsis, septic shock, severe bradycardia DOBUTAMINE – intrope and vasodilator: increases contractility and reduces cardiac work USES: -

Transdermal Drug Delivery Device Including An
(19) TZZ_ZZ¥¥_T (11) EP 1 807 033 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61F 13/02 (2006.01) A61L 15/16 (2006.01) 20.07.2016 Bulletin 2016/29 (86) International application number: (21) Application number: 05815555.7 PCT/US2005/035806 (22) Date of filing: 07.10.2005 (87) International publication number: WO 2006/044206 (27.04.2006 Gazette 2006/17) (54) TRANSDERMAL DRUG DELIVERY DEVICE INCLUDING AN OCCLUSIVE BACKING VORRICHTUNG ZUR TRANSDERMALEN VERABREICHUNG VON ARZNEIMITTELN EINSCHLIESSLICH EINER VERSTOPFUNGSSICHERUNG DISPOSITIF D’ADMINISTRATION TRANSDERMIQUE DE MEDICAMENTS AVEC COUCHE SUPPORT OCCLUSIVE (84) Designated Contracting States: • MANTELLE, Juan AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Miami, FL 33186 (US) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • NGUYEN, Viet SK TR Miami, FL 33176 (US) (30) Priority: 08.10.2004 US 616861 P (74) Representative: Awapatent AB P.O. Box 5117 (43) Date of publication of application: 200 71 Malmö (SE) 18.07.2007 Bulletin 2007/29 (56) References cited: (73) Proprietor: NOVEN PHARMACEUTICALS, INC. WO-A-02/36103 WO-A-97/23205 Miami, FL 33186 (US) WO-A-2005/046600 WO-A-2006/028863 US-A- 4 994 278 US-A- 4 994 278 (72) Inventors: US-A- 5 246 705 US-A- 5 474 783 • KANIOS, David US-A- 5 474 783 US-A1- 2001 051 180 Miami, FL 33196 (US) US-A1- 2002 128 345 US-A1- 2006 034 905 Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Association of Oral Anticoagulants and Proton Pump Inhibitor Cotherapy with Hospitalization for Upper Gastrointestinal Tract Bleeding
Supplementary Online Content Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. doi:10.1001/jama.2018.17242 eAppendix. PPI Co-therapy and Anticoagulant-Related UGI Bleeds This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Appendix: PPI Co-therapy and Anticoagulant-Related UGI Bleeds Table 1A Exclusions: end-stage renal disease Diagnosis or procedure code for dialysis or end-stage renal disease outside of the hospital 28521 – anemia in ckd 5855 – Stage V , ckd 5856 – end stage renal disease V451 – Renal dialysis status V560 – Extracorporeal dialysis V561 – fitting & adjustment of extracorporeal dialysis catheter 99673 – complications due to renal dialysis CPT-4 Procedure Codes 36825 arteriovenous fistula autogenous gr 36830 creation of arteriovenous fistula; 36831 thrombectomy, arteriovenous fistula without revision, autogenous or 36832 revision of an arteriovenous fistula, with or without thrombectomy, 36833 revision, arteriovenous fistula; with thrombectomy, autogenous or nonaut 36834 plastic repair of arteriovenous aneurysm (separate procedure) 36835 insertion of thomas shunt 36838 distal revascularization & interval ligation, upper extremity 36840 insertion mandril 36845 anastomosis mandril 36860 cannula declotting; 36861 cannula declotting; 36870 thrombectomy, percutaneous, arteriovenous -

Wednesday, July 10, 2019 4:00Pm
Wednesday, July 10, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – July 10, 2019 DATE: July 3, 2019 NOTE: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the July meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/SoonerPsych Program Update – Appendix B Action Item – Vote to Prior Authorize Jornay PM™ [Methylphenidate Extended-Release (ER) Capsule], Evekeo ODT™ [Amphetamine Orally Disintegrating Tablet (ODT)], Adhansia XR™ (Methylphenidate ER Capsule), and Sunosi™ (Solriamfetol Tablet) – Appendix C Action Item – Vote to Prior Authorize Balversa™ (Erdafitinib) – Appendix D Action Item – Vote to Prior Authorize Annovera™ (Segesterone Acetate/Ethinyl Estradiol Vaginal System), Bijuva™ (Estradiol/Progesterone Capsule), Cequa™ (Cyclosporine 0.09% Ophthalmic Solution), Corlanor® (Ivabradine Oral Solution), Crotan™ (Crotamiton 10% Lotion), Gloperba® (Colchicine Oral Solution), Glycate® (Glycopyrrolate Tablet), Khapzory™ (Levoleucovorin Injection), Qmiiz™ ODT [Meloxicam Orally Disintegrating Tablet (ODT)], Seconal Sodium™ (Secobarbital -

Table of Contents (PDF)
1 , I..,U i + i !, , i HearBritish Br Heart J: first published as on 1 July 1985. Downloaded from monthly journal of cardiology blished in association with eBritish Cardiac Society Journal m Editorial: strategies for preventing and screening 1 Functionless retained pacing leads in the for coronary heart disease MF Oliver cardiovascular system: a complication of Myocardial infarction and thrombolysis: pacemaker treatment FZerbe, A Ponizynski, electrocardiographic short term and long term WDyszkiewicz, A Ziemiahski, TDzi~gielewski, results using precordial mapping R von Essen, HKrug 76 WSchmidt, R Uebis, B Edelmann, S Effert, J Silny, G Rau 6 Effect of exercise on ventricular response to atrial Rupture of the myocardium: occurrence and risk fibrillation in Wolff-Parkinson-White factors MDellborg, P Held, KSwedberg, A Vedin 1 syndrome JCPCrick, D WDavies, PHolt, P V L Curry, ESowton 80 Effect of xamoterol (ICI 1 1 8587), a new beta, adrenoceptor partial agonist, on resting Intravenous sotalol for the treatment of atrial http://heart.bmj.com/ haemodynamic variables and exercise tolerance in fibrillation and flutter after cardiopulmonary patients with left ventricular dysfunction bypass: comparison with disopyramide and A O Molajo, D HBennett 17 digoxin in a randomised trial TJCampbell, How important is a history of chest pain in TP Gavaghan, JJ Morgan 86 determining the degree of ischaemia in patients Factors relating to the development of with angina pectoris? A A Quyyumi, CM Wright, hypertension after cardiopulmonary bypass L J Mockus, K M Fox 22 TTJ Cooper, TH Clutton-Brock, SNJones, J Tinker, Ischaemic heart disease and prodromes of sudden T Treasure 91 cardiac death: is it possible to identify high risk on October 2, 2021 by guest. -

FACT SHEET for HEALTHCARE PROVIDERS Coronavirus Emergency Use of the Coviage System During the COVID-19 Pandemic Disease 2019 September 24, 2020 (COVID-19)
FACT SHEET FOR HEALTHCARE PROVIDERS Coronavirus Emergency Use of the COViage System During the COVID-19 Pandemic Disease 2019 September 24, 2020 (COVID-19) This Fact Sheet informs you of the significant known and What do I need to know about COVID-19 treatment? potential risks and benefits of the emergency use of the Current information on COVID-19 infection, including COViage System (or “COViage”) for use by healthcare case definitions and information about clinical signs and providers (HCP) in the hospital setting for adult patients symptoms and/or epidemiological criteria, is available on (18 years of age or older who are admitted to the the CDC website listed at the end of this fact sheet. hospital) with confirmed COVID-19 (based on a positive . PCR test result) for the computation of proprietary patient status indices referred to as Respiratory What is the COViage System? Decompensation Status and Hemodynamic Instability The COViage System is a non-interventional software Status as an adjunct to patient monitoring during the program that uses vital signs and patient demographic COVID-19 outbreak. The COViage indices provide HCP data from EMR systems. It uses models derived from with predictive screening information as a diagnostic aid machine learning on patient data from EMRs to calculate to assist with the early identification of COVID-19 the likelihood of the occurrence of certain clinically patients who are likely to be diagnosed with significant events, specifically, hemodynamic instability hemodynamic instability or respiratory decompensation, or respiratory decompensation. It then notifies HCP of which are common complications associated with patients who, according to the algorithm, are expected to COVID-19. -

Desensitization and Selective Down-Regulation of Rat Cardiac '31-Adrenoceptors by Prolonged in Vivo Infusion of T-0509, a 81-Adrenoceptor Full Agonist
Desensitization and Selective Down-Regulation of Rat Cardiac '31-Adrenoceptors by Prolonged In Vivo Infusion of T-0509, a 81-Adrenoceptor Full Agonist Yoji Sato', Satomi Adachi-Akahane', Pablo Prados2, Kazuhiro Imai2 and Taku Nagao'°* 'Departmentof Toxicologyand Pharmacologyand 2Departmentof AnalyticalChemistry , Facultyof PharmaceuticalSciences, Universityof Tokyo,Hongo, Bunkyo-ku,Tokyo 113, Japan ReceivedJune 13, 1995 AcceptedSeptember 18, 1995 ABSTRACT-We studied the effects of prolonged infusion of a selective ~1-adrenoceptor (f3,AR) full agonist, T-0509 [(-)-(R)-1-(3,4-dihydroxyphenyl)-2-[(3,4-dimethoxyphenethyl)amino]ethanol hydrochlo- ride], with regard to its inotropic effect in vivo and cardiac (3AR density. The results were compared with those for isoproterenol. Continuous infusion of isoproterenol at doses of 2.5-40 ,ug/kg/hr, s.c. for 6 days shifted the dose-response curves of isoproterenol (i.v.) for LVdP/dtmx to the right and increased the ED50 values up to fourfold. Isoproterenol infusion at 40 pg/kg/hr reduced the density of both r3,- and ~2ARs by 36010and 43070 respectively, in left ventricular membranes. Following 6-day infusion of T-0509 at doses sufficient to induce a positive inotropic effect (5-40 rig/kg/hr), the ED50 value of T-0509 (i.v.) for LVdP/dtmax was also increased up to fourfold. In contrast to isoproterenol, infusion of T-0509 caused selective down-regulation of (3,ARs by 30% without changing the number of hARs. These results indicate that long-term application of a selective /31AR full agonist causes desensitization to its inotropy in vivo, with subtype-selective down-regulation of ~1ARs in cardiac ventricles. -

Association of Oral Anticoagulants and Proton Pump Inhibitor Cotherapy with Hospitalization for Upper Gastrointestinal Tract Bleeding
Supplementary Online Content Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. doi:10.1001/jama.2018.17242 eAppendix. PPI Co-therapy and Anticoagulant-Related UGI Bleeds This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://edhub.ama-assn.org/ on 09/24/2021 Appendix: PPI Co-therapy and Anticoagulant-Related UGI Bleeds Table 1A Exclusions: end-stage renal disease Diagnosis or procedure code for dialysis or end-stage renal disease outside of the hospital 28521 – anemia in ckd 5855 – Stage V , ckd 5856 – end stage renal disease V451 – Renal dialysis status V560 – Extracorporeal dialysis V561 – fitting & adjustment of extracorporeal dialysis catheter 99673 – complications due to renal dialysis CPT-4 Procedure Codes 36825 arteriovenous fistula autogenous gr 36830 creation of arteriovenous fistula; 36831 thrombectomy, arteriovenous fistula without revision, autogenous or 36832 revision of an arteriovenous fistula, with or without thrombectomy, 36833 revision, arteriovenous fistula; with thrombectomy, autogenous or nonaut 36834 plastic repair of arteriovenous aneurysm (separate procedure) 36835 insertion of thomas shunt 36838 distal revascularization & interval ligation, upper extremity 36840 insertion mandril 36845 anastomosis mandril 36860 cannula declotting; 36861 cannula declotting; 36870 thrombectomy, percutaneous, arteriovenous -

Wednesday, June 12, 2019 4:00Pm
Wednesday, June 12, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – June 12, 2019 DATE: June 5, 2019 Note: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the June meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Use of Angiotensin Converting Enzyme Inhibitor (ACEI)/ Angiotensin Receptor Blocker (ARB) Therapy in Patients with Diabetes and Hypertension (HTN) Mailing Update – Appendix B Action Item – Vote to Prior Authorize Aldurazyme® (Laronidase) and Naglazyme® (Galsulfase) – Appendix C Action Item – Vote to Prior Authorize Plenvu® [Polyethylene Glycol (PEG)-3350/Sodium Ascorbate/Sodium Sulfate/Ascorbic Acid/Sodium Chloride/Potassium Chloride] – Appendix D Action Item – Vote to Prior Authorize Consensi® (Amlodipine/Celecoxib) and Kapspargo™ Sprinkle [Metoprolol Succinate Extended-Release (ER)] – Appendix E Action Item – Vote to Update the Prior Authorization Criteria For H.P. Acthar® Gel (Repository Corticotropin Injection) – Appendix F Action Item – Vote to Prior Authorize Fulphila® (Pegfilgrastim-jmdb), Nivestym™ (Filgrastim-aafi), -
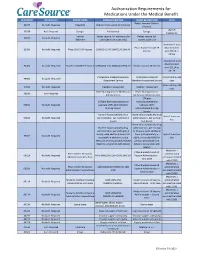
Authorization Requirements for Medications Under
Authorization Requirements for Medications Under the Medical Benefit HCPC/MOD Marketplace BRAND NAME LONG DESCRIPTION SHORT DESCRIPTION Limits Rabies Immune Globulin 90375 No Auth. Required HyperRab Rabies Immune Globulin (Human) (Human) Up to 5 90378 Auth Required Synagis Palivizumab Synagis treatments Imovax Rabies vaccine, for intramuscular Rabies vaccine, for 90675 No Auth. Required Rabavert use (Code price is per 1 mL) intramuscular use Maximum 2 units Pfizer-Biontech Covid-19 Must be billed 91300 No Auth. Required Pfizer COVID-19 Vaccine SARSCOV2 VAC 30MCG/0.3ML IM Vaccine with 0001A or 0002A Maximum 2 units Must be billed 91301 No Auth. Required Moderna COVID-19 Vaccine SARSCOV2 VAC 100MCG/0.5ML IM Moderna Covid-19 Vaccine with 0011A or 0012A Postpartum Maternal Newborn Postpartum Maternal 4 Units Within 180 99501 No Auth. Required Assessment Service Newborn Assessment Service days 4 Units Within 180 99502 No Auth. Required Newborn Assessment Newborn Assessment days Home Nursing Visit for Medication Home Nursing Visit for 99506 Auth Required Administration Medication Administration 17Alpha- 17Alpha-hydroxyprogesterone hydroxyprogesterone 99600 No Auth. Required Caproate (17P) Administration Caproate (17P) Nursing Service Administration Nursing Service Home infusion/specialty drug Home infusion/specialty drug Up to 2 hours per 99601 No Auth. Required administration, per visit (up to 2 administration, per visit (up day hours) to 2 hours) Home infusion/specialty drug Home infusion/specialty drug administration, per visit (up administration, per visit (up to 2 to 2 hours); each additional hours); each additional hour (List hour (List separately in Up to 2 hours per 99602 No Auth. Required separately in addition to code addition to code 99601 for day 99601 for primary procedure) (Use primary procedure) (Use 99602 in conjunction with 99601) 99602 in conjunction with 99601) Maximum 1 Pfizer-Biontech Covid-19 Pfizer COVID-19 Vaccine administration 0001A No Auth. -

Postoperative Vasopressor Usage: a Prospective International Observational Study
SQUEEZE Postoperative vasopressor usage: a prospective international observational study CRF1 PATIENT IDENTIFICATION NUMBER: _____________ PRE-OPERATIVE DATE OF SURGERY: ___________________________ Month and year of birth Sex [m/f] Height [cm] Weight [kg] Clinical Frailty Scale (Rockwood): point 0 to 9. (Will be explained in final CRF) Previous medical history: Coronary Artery Disease: Y/N Cerebrovascular Disease: Y/N Peripheral vascular disease: Y/N Atrial fibrillation: Y/N Heart failure: Y/N Hypertension: Y treated and controlled, Y treated but not controlled, No Diabetes: Takes insulin/managed without insulin/None Chronic liver disease: Y/N Chronic respiratory disease: COPD/other/None Chronic immunosuppression: HIV/other/none Chronic Kidney Disease: No/Yes/Yes and receives renal replacement therapy Long-term steroid use: Y/N Recent/current treatment for cancer (including chemotherapy, radiotherapy, surgery) Regular medications ACE inhibitor: Y and took today/ Y omitted today/N Alpha blocker: Y and took today/ Y omitted today/N Angiotensin Receptor Blocker: Y and took today/ Y omitted today/N Beta blocker: Y and took today/ Y omitted today/N Calcium channel blocker: Y and took today/ Y omitted today Diuretic: Y and took today/ Y omitted today/N Regular NSAIDs: Y/N Regular PPI: Y/N Haemodynamics Measurement in the past 6 months, at least 12h prior to the operating room, at rest: Systolic, Diastolic Heart rate The reading immediately prior to induction of anaesthesia: Systolic, Diastolic Heart rate Laboratory results, most recent (if known -

Cannabinoid CB1 and CB2 Receptors Antagonists AM251 and AM630
Pharmacological Reports 71 (2019) 82–89 Contents lists available at ScienceDirect Pharmacological Reports journal homepage: www.elsevier.com/locate/pharep Original article Cannabinoid CB1 and CB2 receptors antagonists AM251 and AM630 differentially modulate the chronotropic and inotropic effects of isoprenaline in isolated rat atria Jolanta Weresa, Anna Pe˛dzinska-Betiuk, Rafał Kossakowski, Barbara Malinowska* Department of Experimental Physiology and Pathophysiology, Medical University of Bialystok, Białystok, Poland A R T I C L E I N F O A B S T R A C T Article history: Background: Drugs targeting CB1 and CB2 receptors have been suggested to possess therapeutic benefit in Received 28 May 2018 cardiovascular disorders associated with elevated sympathetic tone. Limited data suggest cannabinoid Received in revised form 31 July 2018 ligands interact with postsynaptic β-adrenoceptors. The aim of this study was to examine the effects of Accepted 14 September 2018 CB1 and CB2 antagonists, AM251 and AM630, respectively, at functional cardiac β-adrenoceptors. Available online 17 September 2018 Methods: Experiments were carried out in isolated spontaneously beating right atria and paced left atria where inotropic and chronotropic increases were induced by isoprenaline and selective agonists of β1 and Keywords: β -adrenergic receptors. β-Adrenoceptor 2 Results: We found four different effects of AM251 and AM630 on the cardiostimulatory action of Cannabinoid receptor m AM251 isoprenaline: (1) both CB receptor antagonists 1 M enhanced the isoprenaline-induced increase in atrial AM630 rate, and AM630 1 mM enhanced the inotropic effect of isoprenaline; (2) AM251 1 mM decreased the Atria efficacy of the inotropic effect of isoprenaline; (3) AM251 0.1 and 3 mM and AM630 3 mM reduced the isoprenaline-induced increases in atrial rate; (4) AM630 0.1 and 3 mM enhanced the inotropic effect of isoprenaline, which was not changed by the same concentrations of AM251.