News Release
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nilotinib in Patients with Chronic Myeloid Leukemia: STAT2 Trial in Japan
Haematologica HAEMATOL/2018/194894 Version 3 Haematologica HAEMATOL/2018/194894 Version 3 Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan Naoto Takahashi, Kaichi Nishiwaki, Chiaki Nakaseko, Nobuyuki Aotsuka, Koji Sano, Chikako Ohwada, Jun Kuroki, Hideo Kimura, Michihide Tokuhira, Kinuko Mitani, Kazuhisa Fujikawa, Osamu Iwase, Kohshi Ohishi, Fumihiko Kimura, Tetsuya Fukuda, Sakae Tanosaki, Saori Takahashi, Yoshihiro Kameoka, Hiroyoshi Nishikawa, and Hisashi Wakita Disclosures: 1. This study was supported by research funding from Novartis Pharmaceuticals to N.T. 2. N.T reports grants from Novartis Pharmaceuticals, during the conduct of the study; grants and personal fees from Novartis Pharmaceuticals, grants and personal fees from Otsuka, grants and personal fees from Pfizer, personal fees from Bristol-Myers Squibb, outside the submitted work; K.N reports grants from Zenyaku Kogyo Company, Limited, grants from Chugai Pharmaceutical, grants from Novartis Pharma K.K., grants from Kyowa Hakko Kirin Co, Ltd, grants from Nippon Shinyaku Co, Ltd, outside the submitted work; C.N reports personal fees from Novartis, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Pfizer, grants and personal fees from Takeda pharmaceuticals, grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Otsuka Pharmaceutical, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Asahi Kasei Pharma, grants and personal fees from Shionogi, personal fees from Shire, personal fees from Jannsen, personal fees from Celgene, outside the submitted work; M.T. reports personal fees from Bristol-Myers Squib, personal fees from Pfizer, outside the submitted work; K.M reports grants from Kyowa Hakko Kirin Co. -

1332 Nippon Suisan Kaisha, Ltd. 50 1333 Maruha Nichiro Corp. 500 1605 Inpex Corp
Nikkei Stock Average - Par Value (Update:August/1, 2017) Code Company Name Par Value(Yen) 1332 Nippon Suisan Kaisha, Ltd. 50 1333 Maruha Nichiro Corp. 500 1605 Inpex Corp. 125 1721 Comsys Holdings Corp. 50 1801 Taisei Corp. 50 1802 Obayashi Corp. 50 1803 Shimizu Corp. 50 1808 Haseko Corp. 250 1812 Kajima Corp. 50 1925 Daiwa House Industry Co., Ltd. 50 1928 Sekisui House, Ltd. 50 1963 JGC Corp. 50 2002 Nisshin Seifun Group Inc. 50 2269 Meiji Holdings Co., Ltd. 250 2282 Nh Foods Ltd. 50 2432 DeNA Co., Ltd. 500/3 2501 Sapporo Holdings Ltd. 250 2502 Asahi Group Holdings, Ltd. 50 2503 Kirin Holdings Co., Ltd. 50 2531 Takara Holdings Inc. 50 2768 Sojitz Corp. 500 2801 Kikkoman Corp. 50 2802 Ajinomoto Co., Inc. 50 2871 Nichirei Corp. 100 2914 Japan Tobacco Inc. 50 3086 J.Front Retailing Co., Ltd. 100 3099 Isetan Mitsukoshi Holdings Ltd. 50 3101 Toyobo Co., Ltd. 50 3103 Unitika Ltd. 50 3105 Nisshinbo Holdings Inc. 50 3289 Tokyu Fudosan Holdings Corp. 50 3382 Seven & i Holdings Co., Ltd. 50 3401 Teijin Ltd. 250 3402 Toray Industries, Inc. 50 3405 Kuraray Co., Ltd. 50 3407 Asahi Kasei Corp. 50 3436 SUMCO Corp. 500 3861 Oji Holdings Corp. 50 3863 Nippon Paper Industries Co., Ltd. 500 3865 Hokuetsu Kishu Paper Co., Ltd. 50 4004 Showa Denko K.K. 500 4005 Sumitomo Chemical Co., Ltd. 50 4021 Nissan Chemical Industries, Ltd. 50 4042 Tosoh Corp. 50 4043 Tokuyama Corp. 50 WF-101-E-20170803 Copyright © Nikkei Inc. All rights reserved. 1/5 Nikkei Stock Average - Par Value (Update:August/1, 2017) Code Company Name Par Value(Yen) 4061 Denka Co., Ltd. -

Factset-Top Ten-0521.Xlsm
Pax International Sustainable Economy Fund USD 7/31/2021 Port. Ending Market Value Portfolio Weight ASML Holding NV 34,391,879.94 4.3 Roche Holding Ltd 28,162,840.25 3.5 Novo Nordisk A/S Class B 17,719,993.74 2.2 SAP SE 17,154,858.23 2.1 AstraZeneca PLC 15,759,939.73 2.0 Unilever PLC 13,234,315.16 1.7 Commonwealth Bank of Australia 13,046,820.57 1.6 L'Oreal SA 10,415,009.32 1.3 Schneider Electric SE 10,269,506.68 1.3 GlaxoSmithKline plc 9,942,271.59 1.2 Allianz SE 9,890,811.85 1.2 Hong Kong Exchanges & Clearing Ltd. 9,477,680.83 1.2 Lonza Group AG 9,369,993.95 1.2 RELX PLC 9,269,729.12 1.2 BNP Paribas SA Class A 8,824,299.39 1.1 Takeda Pharmaceutical Co. Ltd. 8,557,780.88 1.1 Air Liquide SA 8,445,618.28 1.1 KDDI Corporation 7,560,223.63 0.9 Recruit Holdings Co., Ltd. 7,424,282.72 0.9 HOYA CORPORATION 7,295,471.27 0.9 ABB Ltd. 7,293,350.84 0.9 BASF SE 7,257,816.71 0.9 Tokyo Electron Ltd. 7,049,583.59 0.9 Munich Reinsurance Company 7,019,776.96 0.9 ASSA ABLOY AB Class B 6,982,707.69 0.9 Vestas Wind Systems A/S 6,965,518.08 0.9 Merck KGaA 6,868,081.50 0.9 Iberdrola SA 6,581,084.07 0.8 Compagnie Generale des Etablissements Michelin SCA 6,555,056.14 0.8 Straumann Holding AG 6,480,282.66 0.8 Atlas Copco AB Class B 6,194,910.19 0.8 Deutsche Boerse AG 6,186,305.10 0.8 UPM-Kymmene Oyj 5,956,283.07 0.7 Deutsche Post AG 5,851,177.11 0.7 Enel SpA 5,808,234.13 0.7 AXA SA 5,790,969.55 0.7 Nintendo Co., Ltd. -
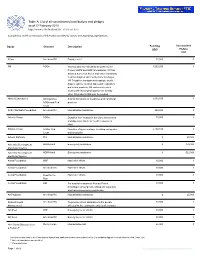
Table A: List of All Commitments/Contributions and Pledges As of 17 February 2010 (Table Ref: R10)
Table A: List of all commitments/contributions and pledges as of 17 February 2010 http://www.reliefweb.int/fts (Table ref: R10) Compiled by OCHA on the basis of information provided by donors and appealing organizations. Donor Channel Description Funding Uncommitted USD Pledges USD 3Com American RC Disaster relief 10,000 0 3M NGOs Working with key humanitarian partners like 1,000,000 0 Project HOPE and MAP International, 3M has donated numerous boxes and cases containing medical supplies such as Nexcare bandages, 3M Tegaderm transparent dressings, sterile drapes, splints, medical tapes and respiratory protection products. 3M continues to work closely with its nonprofit partners to identify other 3M products that may be needed. Abbott Laboratories UN Agencies, In-kind: Donations of medicines and nutritional 1,000,000 0 NGOs and Red products Cross ACE Charitable Foundation American RC Humanitarian assistance 250,000 0 Actavis Group NGOs Donation from Actavis in the US to Americares 10,000 0 and Operation Smile for health response in Haiti. Actavis Group NGOs; Red Donation of generic drugs, including analgesics 2,100,840 0 Cross and antibiotics. Advent Software PIH Humanitarian assistance 0 25,000 Adventist Development ADRA-Haiti Emergency assistance 0 478,000 and Relief Agency Adventist Development ADRA-Haiti Emergency assistance 0 522,000 and Relief Agency Aetna Foundation MSF Haiti relief efforts 10,000 0 Aetna Foundation American RC Haiti relief efforts 10,000 0 Aetna Foundation Food for the Haiti relief efforts 10,000 0 Poor Aetna Foundation UM For medical missions to Port-au-Prince, 10,000 0 including neurosurgeons, orthopedic surgeons and trauma/emergency physicians. -

Integrated Report 2016 Year Ended March 31, 2016 Strengths That Increase Supporting Sustainable Creating Long-Term Value Our Value to Society Manufacturing Processes
Integrated Report 2016 Year ended March 31, 2016 Strengths that increase Supporting sustainable Creating long-term value our value to society manufacturing processes The Company Policy of Shionogi Contents Strengths that increase our value to society The Company Policy of Shionogi (Established in 1957) Creating long-term value Supporting sustainable manufacturing processes Data Section Editorial Policy This Integrated Report provides a wide range of information to give shareholders, Forward-looking Statements investors and other stakeholders a deeper understanding of the Shionogi Group’s This report contains forward-looking statements. These statements are based on corporate value. In addition to financial data, readers can access information expectations in light of the information currently available, assumptions that are about management strategy and the Group’s governance, social and environmen- subject to risks, and uncertainties which could cause actual results to differ tal activities. materially from these statements. Risks and uncertainties include general domestic and international economic Period Under Review conditions, such as general industry and market conditions, and changes of Fiscal 2015 (April 1, 2015–March 31, 2016) interest rates and currency exchange rates. Certain activities continuing after fiscal 2015 are also included. These risks and uncertainties particularly apply to forward-looking statements concerning existing products and those under development. Product risks and Scope and Organization uncertainties include, but are not limited to, completion and discontinuation of This Integrated Report encompasses the activities of Shionogi & Co., Ltd. and 37 clinical trials; obtaining regulatory approvals; claims and concerns about product consolidated subsidiaries. safety and efficacy; technological advances; adverse outcome of important In this report, “Shionogi” refers to Shionogi & Co., Ltd. -

Translation for Reference Only Ticker Code: 4507 to All Shareholders June 5, 2018
Translation for reference only Ticker Code: 4507 To All Shareholders June 5, 2018 Notice of Convocation of the 153rd Annual General Meeting of Shareholders The 153rd Annual General Meeting of Shareholders will be convened at the time and location listed below. On behalf of the directors of the Company, we cordially invite you to attend this shareholders’ meeting. If you are unable to attend, you can exercise your voting rights with the proxy form on the back of this notice. If you wish to vote by using the proxy form, you are kindly requested to take the time to review the reference information provided below and exercise it by 5:00 p.m., Tuesday June 19, 2018.1 Yours faithfully, Isao Teshirogi Representative director and president and CEO Shionogi & Co., Ltd. 1-8, Doshomachi 3-chome, Chuo-ku, Osaka 541-0045, Japan Annual General Meeting of Shareholders 1. Date and time: 10:00 a.m., Wednesday, June 20, 2018 2. Location: HERBIS HALL 5-25, Umeda 2-chome, Kita-ku, Osaka 530-0001, Japan 3. Agenda: Items to report: 1. The Business Report, the Consolidated Financial Statements and the Non-Consolidated Financial Statements for the 153rd Fiscal Term (year ended March 31, 2018) 2. The Audit Report of the Consolidated Financial Statements for the 153rd Fiscal Term (year ended March 31, 2018) by the Accounting Auditor and the Board of Corporate Auditors Items for resolution: Proposal No. 1: Appropriation of Surplus Proposal No. 2: Amendments to the Articles of Incorporation Proposal No. 3: Election of Six (6) Directors Proposal No. -

Introduction
Hypertension Research (2014) 37, 256–259 & 2014 The Japanese Society of Hypertension All rights reserved 0916-9636/14 www.nature.com/hr GUIDELINES (JSH 2014) Introduction Hypertension Research (2014) 37, 256–259; doi:10.1038/hr.2014.18 The Japanese Society of Hypertension revised the Japanese Society of JSH 2014 should also be used by health nurses, nurses, dietitians and Hypertension Guidelines for the Management of Hypertension in staff responsible for team practice for hypertension management. 2009 (JSH 2009) and published the JSH 2014. Basically, the JSH 2014 Therefore, in addition to specialists in hypertension, the members of was prepared according to strategies to prepare the JSH 2009 and the The Japan Association of Medical Practitioners, The Japanese Society ‘Guidance for the Preparation of Treatment Guidelines in 2007’ of Clinical Pharmacology and Therapeutics, Japan Pharmaceutical established by the Medical Information Network Distribution Service. Association, Japanese Society of Clinical Nutrition, and Patient In the ‘Introduction’ section, methods to prepare the JSH 2014 are Corporation belong to the Japanese Society of Hypertension Com- introduced. mittee for Guidelines for the Management of Hypertension. With regard to the affiliations of the committee members, their occupation, 1. OBJECTIVE AND SUBJECTS OF THE JSH 2014 affiliated corporations and positions are described. Hypertension causes stroke (cerebral infarction, cerebral hemorrhage, subarachnoidal hemorrhage), heart disease (coronary artery disease, 2. COMPOSITION OF THE JAPANESE SOCIETY OF cardiac hypertrophy, heart failure), kidney disease (nephrosclerosis) HYPERTENSION COMMITTEE FOR GUIDELINES FOR THE and macrovascular disease. Therefore, the primary objective MANAGEMENT OF HYPERTENSION of the JSH 2014 is to present standard treatment to prevent the The Japanese Society of Hypertension Guidelines for the Management onset/progression of hypertensive complications of the brain/heart/ of Hypertension is official. -

Annual Report 2016 for the Year Ended December 31, 2016
Annual Report 2016 For the year ended December 31, 2016 Leaping Forward Contents Review of Operations Editorial Policy 02 (Contents/Editorial Policy) 18 (Pharmaceuticals Business/Bio-chemicals Business) This is our Annual Report for 2016. The environment surrounding the pharmaceuticals industry is rapidly changing, and there Our Philosophy Corporate Governance is a movement to dramatically revise the framework that has (Management Philosophy/Core Values) (Board of Directors/Corporate Governance) 03 31 underpinned the industry thus far. Despite this environment, “human resources” and “technology” will continue to be the Who we are Compliance baseline for innovation at Kyowa Hakko Kirin Group, which 04 (Group Structure/Business Model) 35 (Compliance/Risk Management) aspires to realize health and well-being. In the special features, we describe our human resource development that is promoting our globalization and our production technology for biopharmaceuticals. Niro Sakamoto, Executive Officer, Director of What we do Dialogue Please deepen your understanding of our group, which is taking Corporate Communications 06 (FY2016-2020 Mid-term Business Plan) 38 (Vice President × Outside Directors) initiatives to make the leap forward to become a Global Specialty Department Pharmaceutical Company (GSP). High Top Message Financial Information 07 41 Importance to Stakeholders Annual Report (PDF version) http://ir.kyowa-kirin.com/en/library/annual_report.html Special Feature Kyowa Hakko Kirin Website Special Feature 1: Human Resource Development Investor -

Pasetocin and Sawacillin
Pasetocin® and Sawacillin®: Joint Application Seeking Approval for Additional Indication for Helicobacter pylori Eradication by Triple Therapy with Proton Pump Inhibitors and either Clarithromycin or Metronidazole Tokyo, August 31, 2012 - Kyowa Hakko Kirin Co., Ltd. (“Kyowa Hakko Kirin”; Tokyo:4151; President and CEO: Nobuo Hanai) and Astellas Pharma Inc. (“Astellas Pharma”; Tokyo:4503; President and CEO: Yoshihiko Hatanaka) announced today that they have submitted a joint application* to Japan’s Ministry of Health, Labour and Welfare seeking approval of Helicobacter pylori (“H. pylori”) gastritis as an additional indication for H. pylori eradication by triple therapy including amoxicillin hydrate (generic name; brand names: “Pasetocin® Capsules 125 and 250; Pasetocin® Tablets 250”, “Sawacillin® Capsules 125 and 250; Sawacillin® Tablets 250” and one other brand). This concomitant therapy consists of a proton pump inhibitor (lansoprazole, omeprazole, rabeprazole sodium and esomeprazole magnesium hydrate, generic name; marketed under five brand names), amoxicillin hydrate, and either clarithromycin (generic name; marketed under two brand names) or metronidazole (generic name; marketed under one brand name). H. pylori gastritis is also known as chronic active gastritis due to the fact that it causes histological gastric mucosal injury as a result of the persistent infiltration of inflammatory cells in the gastric mucosa due to H. pylori infection, and is believed to be associated with the development of various H. pylori-related diseases such as gastric and duodenal ulcers. However, under the Japanese National Health Insurance (NHI) system, the approved indications for eradication of H. pylori are currently limited to gastric and duodenal ulcers, gastric MALT lymphoma, idiopathic thrombocytopenic purpura, and the stomach after endoscopic resection of early stage gastric cancer. -

Notice of Convocation of the 151St Annual General Meeting of Shareholders
Translation for reference only Ticker Code: 4507 To All Shareholders June 1, 2016 Notice of Convocation of the 151st Annual General Meeting of Shareholders The 151st Annual General Meeting of Shareholders will be convened at the time and location listed below. On behalf of the directors of the Company, we cordially invite you to attend this shareholders’ meeting. If you are unable to attend, you can exercise your voting rights with the proxy form on the back of this notice. If you wish to vote by using the proxy form, you are kindly requested to take the time to review the reference information provided below and exercise it by 5:00 p.m., Wednesday June 22, 2016.1 Yours faithfully, Isao Teshirogi Representative director and president and CEO Shionogi & Co., Ltd. 1-8, Doshomachi 3-chome, Chuo-ku, Osaka 541-0045, Japan Annual General Meeting of Shareholders 1. Date and time: 10:00 a.m., Thursday, June 23, 2016 2. Location: HERBIS HALL 5-25, Umeda 2-chome, Kita-ku, Osaka 530-0001, Japan 3. Agenda: Items to report: 1. The Business Report, the Consolidated Financial Statements and the Non-Consolidated Financial Statements for the 151st Fiscal Term (year ended March 31, 2016) 2. The Audit Report of the Consolidated Financial Statements for the 151st Fiscal Term (year ended March 31, 2016) by the Accounting Auditor and the Board of Corporate Auditors Items for resolution: Proposal No. 1: Appropriation of Surplus Proposal No. 2: Election of Six (6) Directors Proposal No. 3: Election of Two (2) Corporate Auditors 4. Exercise of voting rights: You are kindly requested to review “How to Exercise Your Voting Rights” on pages 2 and 3 before exercising your voting rights. -

572KB/2Pages
Introduction/ Chapter I Chapter II Chapter III Chapter IV President’s Message Value Creation Story of MinebeaMitsumi Financial Strategy and Capital Policy Initiatives for Value Creation Initiatives to Support Value Creation List of Officers (As of August 2020) ■ Directors Attendance at the Board of Directors Meeting Attendance at the Board of Directors Meeting 100% (12/12) 100% (12/12) Representative Director, CEO & COO Representative Director, Vice Chairman Yoshihisa Kainuma Shigeru Moribe Apr. 1983 Member of Daini Tokyo Bar Association Mar. 1980 Joined MITSUMI ELECTRIC CO., LTD. Dec. 1988 Director, General Manager of Legal Department of the Company May 1990 General Manager of Development Headquarters, MITSUMI ELECTRIC CO., LTD. Sep. 1989 Member of New York State Bar Association Apr. 1991 Director, Head of Singapore branch, MITSUMI ELECTRIC CO., LTD. Dec. 1992 Managing Director and Deputy General Manager of Operations Headquarters Apr. 1994 Managing Director, MITSUMI ELECTRIC CO., LTD. Dec. 1994 Senior Managing Director, General Manager of European and American Regional Oct. 1999 Senior Managing Director, General Manager of Sales Headquarters, MITSUMI Sales Headquarters, Deputy General Manager of Operations Headquarters ELECTRIC CO., LTD. Jun. 2003 Director, Senior Managing Executive Officer Apr. 2002 Representative Director, President, MITSUMI ELECTRIC CO., LTD. Apr. 2009 Representative Director, President and Chief Executive Officer Jan. 2017 Adviser of the Company Jan. 2017 Director, Chairman of the Board of Directors, MITSUMI ELECTRIC CO., LTD. Apr. 2017 Director, Chairman of the Board of Directors, MITSUMI ELECTRIC CO., LTD. (Present) Jun. 2017 Representative Director, CEO & COO (Present) Jun. 2017 Representative Director, Vice Chairman (Present) Aug. 2019 Representative Director, Chairman of the Board of Directors, U-Shin Ltd. -

Ulcerative Colitis Outcomes Research in Japan
Open access Protocol BMJ Open: first published as 10.1136/bmjopen-2019-030134 on 8 September 2019. Downloaded from Ulcerative colitis outcomes research in Japan: protocol for an observational prospective cohort study of YOURS (YOu and Ulcerative colitis: Registry and Social network) Hajime Yamazaki, 1 Katsuyoshi Matsuoka,2 Jovelle Fernandez,3 Toshifumi Hibi,4 Mamoru Watanabe,5,6 Tadakazu Hisamatsu,7 Shunichi Fukuhara1 To cite: Yamazaki H, ABSTRACT Strengths and limitations of this study Matsuoka K, Fernandez J, et al. Introduction Ulcerative colitis (UC) is a chronic Ulcerative colitis outcomes inflammatory disease that mainly affects the colon in research in Japan: protocol for ► The YOu and Ulcerative colitis: Registry and Social young patients. Typical symptoms of UC are bloody an observational prospective network (YOURS) study is a large-scale, long-term, cohort study of YOURS (YOu diarrhoea and faecal urgency, which disturb the quality prospective observational study to explore optimal and Ulcerative colitis: Registry of life (QOL) of patients, and intractable UC leads to ulcerative colitis management for improving rele- and Social network). BMJ Open hospitalisation and colectomy. To improve relevant vant outcomes. 2019;9:e030134. doi:10.1136/ outcomes such as symptoms, QOL and colectomy, many ► The YOURS study reflects the real clinical setting bmjopen-2019-030134 clinical questions need to be resolved regarding what because any concomitant drugs and therapies are ► Prepublication history for the ideal lifestyle, psychosocial burden and optimal allowed. this paper is available online. practice patterns are. In this YOu and Ulcerative colitis: ► The YOURS study can be expanded for subsequent To view these files, please visit Registry and Social network (YOURS) study, we will surveys because patients and healthcare profes- the journal online (http:// dx.