Results Presentation Fiscal 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nilotinib in Patients with Chronic Myeloid Leukemia: STAT2 Trial in Japan
Haematologica HAEMATOL/2018/194894 Version 3 Haematologica HAEMATOL/2018/194894 Version 3 Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan Naoto Takahashi, Kaichi Nishiwaki, Chiaki Nakaseko, Nobuyuki Aotsuka, Koji Sano, Chikako Ohwada, Jun Kuroki, Hideo Kimura, Michihide Tokuhira, Kinuko Mitani, Kazuhisa Fujikawa, Osamu Iwase, Kohshi Ohishi, Fumihiko Kimura, Tetsuya Fukuda, Sakae Tanosaki, Saori Takahashi, Yoshihiro Kameoka, Hiroyoshi Nishikawa, and Hisashi Wakita Disclosures: 1. This study was supported by research funding from Novartis Pharmaceuticals to N.T. 2. N.T reports grants from Novartis Pharmaceuticals, during the conduct of the study; grants and personal fees from Novartis Pharmaceuticals, grants and personal fees from Otsuka, grants and personal fees from Pfizer, personal fees from Bristol-Myers Squibb, outside the submitted work; K.N reports grants from Zenyaku Kogyo Company, Limited, grants from Chugai Pharmaceutical, grants from Novartis Pharma K.K., grants from Kyowa Hakko Kirin Co, Ltd, grants from Nippon Shinyaku Co, Ltd, outside the submitted work; C.N reports personal fees from Novartis, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Pfizer, grants and personal fees from Takeda pharmaceuticals, grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Otsuka Pharmaceutical, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Asahi Kasei Pharma, grants and personal fees from Shionogi, personal fees from Shire, personal fees from Jannsen, personal fees from Celgene, outside the submitted work; M.T. reports personal fees from Bristol-Myers Squib, personal fees from Pfizer, outside the submitted work; K.M reports grants from Kyowa Hakko Kirin Co. -

1332 Nippon Suisan Kaisha, Ltd. 50 1333 Maruha Nichiro Corp. 500 1605 Inpex Corp
Nikkei Stock Average - Par Value (Update:August/1, 2017) Code Company Name Par Value(Yen) 1332 Nippon Suisan Kaisha, Ltd. 50 1333 Maruha Nichiro Corp. 500 1605 Inpex Corp. 125 1721 Comsys Holdings Corp. 50 1801 Taisei Corp. 50 1802 Obayashi Corp. 50 1803 Shimizu Corp. 50 1808 Haseko Corp. 250 1812 Kajima Corp. 50 1925 Daiwa House Industry Co., Ltd. 50 1928 Sekisui House, Ltd. 50 1963 JGC Corp. 50 2002 Nisshin Seifun Group Inc. 50 2269 Meiji Holdings Co., Ltd. 250 2282 Nh Foods Ltd. 50 2432 DeNA Co., Ltd. 500/3 2501 Sapporo Holdings Ltd. 250 2502 Asahi Group Holdings, Ltd. 50 2503 Kirin Holdings Co., Ltd. 50 2531 Takara Holdings Inc. 50 2768 Sojitz Corp. 500 2801 Kikkoman Corp. 50 2802 Ajinomoto Co., Inc. 50 2871 Nichirei Corp. 100 2914 Japan Tobacco Inc. 50 3086 J.Front Retailing Co., Ltd. 100 3099 Isetan Mitsukoshi Holdings Ltd. 50 3101 Toyobo Co., Ltd. 50 3103 Unitika Ltd. 50 3105 Nisshinbo Holdings Inc. 50 3289 Tokyu Fudosan Holdings Corp. 50 3382 Seven & i Holdings Co., Ltd. 50 3401 Teijin Ltd. 250 3402 Toray Industries, Inc. 50 3405 Kuraray Co., Ltd. 50 3407 Asahi Kasei Corp. 50 3436 SUMCO Corp. 500 3861 Oji Holdings Corp. 50 3863 Nippon Paper Industries Co., Ltd. 500 3865 Hokuetsu Kishu Paper Co., Ltd. 50 4004 Showa Denko K.K. 500 4005 Sumitomo Chemical Co., Ltd. 50 4021 Nissan Chemical Industries, Ltd. 50 4042 Tosoh Corp. 50 4043 Tokuyama Corp. 50 WF-101-E-20170803 Copyright © Nikkei Inc. All rights reserved. 1/5 Nikkei Stock Average - Par Value (Update:August/1, 2017) Code Company Name Par Value(Yen) 4061 Denka Co., Ltd. -

Introduction
Hypertension Research (2014) 37, 256–259 & 2014 The Japanese Society of Hypertension All rights reserved 0916-9636/14 www.nature.com/hr GUIDELINES (JSH 2014) Introduction Hypertension Research (2014) 37, 256–259; doi:10.1038/hr.2014.18 The Japanese Society of Hypertension revised the Japanese Society of JSH 2014 should also be used by health nurses, nurses, dietitians and Hypertension Guidelines for the Management of Hypertension in staff responsible for team practice for hypertension management. 2009 (JSH 2009) and published the JSH 2014. Basically, the JSH 2014 Therefore, in addition to specialists in hypertension, the members of was prepared according to strategies to prepare the JSH 2009 and the The Japan Association of Medical Practitioners, The Japanese Society ‘Guidance for the Preparation of Treatment Guidelines in 2007’ of Clinical Pharmacology and Therapeutics, Japan Pharmaceutical established by the Medical Information Network Distribution Service. Association, Japanese Society of Clinical Nutrition, and Patient In the ‘Introduction’ section, methods to prepare the JSH 2014 are Corporation belong to the Japanese Society of Hypertension Com- introduced. mittee for Guidelines for the Management of Hypertension. With regard to the affiliations of the committee members, their occupation, 1. OBJECTIVE AND SUBJECTS OF THE JSH 2014 affiliated corporations and positions are described. Hypertension causes stroke (cerebral infarction, cerebral hemorrhage, subarachnoidal hemorrhage), heart disease (coronary artery disease, 2. COMPOSITION OF THE JAPANESE SOCIETY OF cardiac hypertrophy, heart failure), kidney disease (nephrosclerosis) HYPERTENSION COMMITTEE FOR GUIDELINES FOR THE and macrovascular disease. Therefore, the primary objective MANAGEMENT OF HYPERTENSION of the JSH 2014 is to present standard treatment to prevent the The Japanese Society of Hypertension Guidelines for the Management onset/progression of hypertensive complications of the brain/heart/ of Hypertension is official. -

Annual Report 2016 for the Year Ended December 31, 2016
Annual Report 2016 For the year ended December 31, 2016 Leaping Forward Contents Review of Operations Editorial Policy 02 (Contents/Editorial Policy) 18 (Pharmaceuticals Business/Bio-chemicals Business) This is our Annual Report for 2016. The environment surrounding the pharmaceuticals industry is rapidly changing, and there Our Philosophy Corporate Governance is a movement to dramatically revise the framework that has (Management Philosophy/Core Values) (Board of Directors/Corporate Governance) 03 31 underpinned the industry thus far. Despite this environment, “human resources” and “technology” will continue to be the Who we are Compliance baseline for innovation at Kyowa Hakko Kirin Group, which 04 (Group Structure/Business Model) 35 (Compliance/Risk Management) aspires to realize health and well-being. In the special features, we describe our human resource development that is promoting our globalization and our production technology for biopharmaceuticals. Niro Sakamoto, Executive Officer, Director of What we do Dialogue Please deepen your understanding of our group, which is taking Corporate Communications 06 (FY2016-2020 Mid-term Business Plan) 38 (Vice President × Outside Directors) initiatives to make the leap forward to become a Global Specialty Department Pharmaceutical Company (GSP). High Top Message Financial Information 07 41 Importance to Stakeholders Annual Report (PDF version) http://ir.kyowa-kirin.com/en/library/annual_report.html Special Feature Kyowa Hakko Kirin Website Special Feature 1: Human Resource Development Investor -

Pasetocin and Sawacillin
Pasetocin® and Sawacillin®: Joint Application Seeking Approval for Additional Indication for Helicobacter pylori Eradication by Triple Therapy with Proton Pump Inhibitors and either Clarithromycin or Metronidazole Tokyo, August 31, 2012 - Kyowa Hakko Kirin Co., Ltd. (“Kyowa Hakko Kirin”; Tokyo:4151; President and CEO: Nobuo Hanai) and Astellas Pharma Inc. (“Astellas Pharma”; Tokyo:4503; President and CEO: Yoshihiko Hatanaka) announced today that they have submitted a joint application* to Japan’s Ministry of Health, Labour and Welfare seeking approval of Helicobacter pylori (“H. pylori”) gastritis as an additional indication for H. pylori eradication by triple therapy including amoxicillin hydrate (generic name; brand names: “Pasetocin® Capsules 125 and 250; Pasetocin® Tablets 250”, “Sawacillin® Capsules 125 and 250; Sawacillin® Tablets 250” and one other brand). This concomitant therapy consists of a proton pump inhibitor (lansoprazole, omeprazole, rabeprazole sodium and esomeprazole magnesium hydrate, generic name; marketed under five brand names), amoxicillin hydrate, and either clarithromycin (generic name; marketed under two brand names) or metronidazole (generic name; marketed under one brand name). H. pylori gastritis is also known as chronic active gastritis due to the fact that it causes histological gastric mucosal injury as a result of the persistent infiltration of inflammatory cells in the gastric mucosa due to H. pylori infection, and is believed to be associated with the development of various H. pylori-related diseases such as gastric and duodenal ulcers. However, under the Japanese National Health Insurance (NHI) system, the approved indications for eradication of H. pylori are currently limited to gastric and duodenal ulcers, gastric MALT lymphoma, idiopathic thrombocytopenic purpura, and the stomach after endoscopic resection of early stage gastric cancer. -

572KB/2Pages
Introduction/ Chapter I Chapter II Chapter III Chapter IV President’s Message Value Creation Story of MinebeaMitsumi Financial Strategy and Capital Policy Initiatives for Value Creation Initiatives to Support Value Creation List of Officers (As of August 2020) ■ Directors Attendance at the Board of Directors Meeting Attendance at the Board of Directors Meeting 100% (12/12) 100% (12/12) Representative Director, CEO & COO Representative Director, Vice Chairman Yoshihisa Kainuma Shigeru Moribe Apr. 1983 Member of Daini Tokyo Bar Association Mar. 1980 Joined MITSUMI ELECTRIC CO., LTD. Dec. 1988 Director, General Manager of Legal Department of the Company May 1990 General Manager of Development Headquarters, MITSUMI ELECTRIC CO., LTD. Sep. 1989 Member of New York State Bar Association Apr. 1991 Director, Head of Singapore branch, MITSUMI ELECTRIC CO., LTD. Dec. 1992 Managing Director and Deputy General Manager of Operations Headquarters Apr. 1994 Managing Director, MITSUMI ELECTRIC CO., LTD. Dec. 1994 Senior Managing Director, General Manager of European and American Regional Oct. 1999 Senior Managing Director, General Manager of Sales Headquarters, MITSUMI Sales Headquarters, Deputy General Manager of Operations Headquarters ELECTRIC CO., LTD. Jun. 2003 Director, Senior Managing Executive Officer Apr. 2002 Representative Director, President, MITSUMI ELECTRIC CO., LTD. Apr. 2009 Representative Director, President and Chief Executive Officer Jan. 2017 Adviser of the Company Jan. 2017 Director, Chairman of the Board of Directors, MITSUMI ELECTRIC CO., LTD. Apr. 2017 Director, Chairman of the Board of Directors, MITSUMI ELECTRIC CO., LTD. (Present) Jun. 2017 Representative Director, CEO & COO (Present) Jun. 2017 Representative Director, Vice Chairman (Present) Aug. 2019 Representative Director, Chairman of the Board of Directors, U-Shin Ltd. -

Ulcerative Colitis Outcomes Research in Japan
Open access Protocol BMJ Open: first published as 10.1136/bmjopen-2019-030134 on 8 September 2019. Downloaded from Ulcerative colitis outcomes research in Japan: protocol for an observational prospective cohort study of YOURS (YOu and Ulcerative colitis: Registry and Social network) Hajime Yamazaki, 1 Katsuyoshi Matsuoka,2 Jovelle Fernandez,3 Toshifumi Hibi,4 Mamoru Watanabe,5,6 Tadakazu Hisamatsu,7 Shunichi Fukuhara1 To cite: Yamazaki H, ABSTRACT Strengths and limitations of this study Matsuoka K, Fernandez J, et al. Introduction Ulcerative colitis (UC) is a chronic Ulcerative colitis outcomes inflammatory disease that mainly affects the colon in research in Japan: protocol for ► The YOu and Ulcerative colitis: Registry and Social young patients. Typical symptoms of UC are bloody an observational prospective network (YOURS) study is a large-scale, long-term, cohort study of YOURS (YOu diarrhoea and faecal urgency, which disturb the quality prospective observational study to explore optimal and Ulcerative colitis: Registry of life (QOL) of patients, and intractable UC leads to ulcerative colitis management for improving rele- and Social network). BMJ Open hospitalisation and colectomy. To improve relevant vant outcomes. 2019;9:e030134. doi:10.1136/ outcomes such as symptoms, QOL and colectomy, many ► The YOURS study reflects the real clinical setting bmjopen-2019-030134 clinical questions need to be resolved regarding what because any concomitant drugs and therapies are ► Prepublication history for the ideal lifestyle, psychosocial burden and optimal allowed. this paper is available online. practice patterns are. In this YOu and Ulcerative colitis: ► The YOURS study can be expanded for subsequent To view these files, please visit Registry and Social network (YOURS) study, we will surveys because patients and healthcare profes- the journal online (http:// dx. -

Annual Reports 2008/3
KYOWA HAKKO KOGYO CO., LTD. A New Beginning ANNUAL REPORT 2008 KYOWA HAKKO ANNUAL REPORT 2008 Year ended March 31, 2008 About the Company Kyowa Hakko Kogyo Co., Ltd., is an R&D-based company with special strengths in biotechnology. The Company is dedicated to the creation of new value in the life sciences, especially in its core business segments of Pharmaceuticals and Bio-Chemicals, and strives to contribute to the health and well-being of people around the world. Becoming a member of the Kirin Group on April 1, 2008, Kyowa Hakko is seeking new heights by aggressively promoting its proprietary technologies. In Pharmaceuticals, the Company has actively engaged in the R&D, production, and sale of pharmaceuticals that address needs in such areas as cancer, allergies, renal anemia, and hypertension. Utilizing leading-edge biotechnologies—particularly antibody technologies—we are aiming to be a global specialty pharma that creates innovative pharmaceuticals. In Bio-Chemicals, Kyowa Hakko is a global leader in fermented bulk products, such as amino acids and nucleic acids. In Chemical operations, the Company is expanding lineups of specialty chemicals that contribute to environmental conservation. In Food operations, the Company draws on its fermentation and other original technologies to distinguish itself from competitors in the development of food ingredients, especially natural seasonings. NOTE TO PERFORMANCE FORECASTS Forecasts contained in the Annual Report 2008 represent judgments based on information available as of June 24, 2008. It should be noted that there is a possibility that actual results could differ significantly due to such factors as exchange rate fluctuations. -

CDP Japan Water Security Report 2019
CDP Japan Water Security Report 2019 On behalf of 525 institutional investors with assets of USD 96 trillion CDP Japan Water Security Report 2019 | 2020 March Report writer Contents CDP Foreword 3 Report Writer Foreword 4 Water Security A List 2019 6 Scoring 7 Stories of Change 8 - Kao Corporation - Japan Tobacco Inc. Executive Summary 12 Response to CDP’s Water Security Questionnaire 14 Appendix 22 - CDP Water Security 2019 Japanese companies Please note that the names of companies in the text do not indicate their corporate status. Important Notice The contents of this report may be used by anyone providing acknowledgment is given to CDP. This does not represent a license to repackage or resell any of the data reported to CDP or the contributing authors and presented in this report. If you intend to repackage or resell any of the contents of this report, you need to obtain express permission from CDP before doing so. CDP has prepared the data and analysis in this report based on responses to the CDP 2019 information request. No representation or warranty (express or implied) is given by CDP as to the accuracy or completeness of the information and opinions contained in this report. You should not act upon the information contained in this publication without obtaining specific professional advice. To the extent permitted by law, CDP does not accept or assume any liability, responsibility or duty of care for any consequences of you or anyone else acting, or refraining to act, in reliance on the information contained in this report or for any decision based on it. -
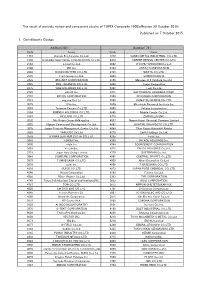
Published on 7 October 2015 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 30 October 2015) Published on 7 October 2015 1. Constituents Change Addition( 80 ) Deletion( 72 ) Code Issue Code Issue 1712 Daiseki Eco.Solution Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 1930 HOKURIKU ELECTRICAL CONSTRUCTION CO.,LTD. 2410 CAREER DESIGN CENTER CO.,LTD. 2183 Linical Co.,Ltd. 2692 ITOCHU-SHOKUHIN Co.,Ltd. 2198 IKK Inc. 2733 ARATA CORPORATION 2266 ROKKO BUTTER CO.,LTD. 2735 WATTS CO.,LTD. 2372 I'rom Group Co.,Ltd. 3004 SHINYEI KAISHA 2428 WELLNET CORPORATION 3159 Maruzen CHI Holdings Co.,Ltd. 2445 SRG TAKAMIYA CO.,LTD. 3204 Toabo Corporation 2475 WDB HOLDINGS CO.,LTD. 3361 Toell Co.,Ltd. 2729 JALUX Inc. 3371 SOFTCREATE HOLDINGS CORP. 2767 FIELDS CORPORATION 3396 FELISSIMO CORPORATION 2931 euglena Co.,Ltd. 3580 KOMATSU SEIREN CO.,LTD. 3079 DVx Inc. 3636 Mitsubishi Research Institute,Inc. 3093 Treasure Factory Co.,LTD. 3639 Voltage Incorporation 3194 KIRINDO HOLDINGS CO.,LTD. 3669 Mobile Create Co.,Ltd. 3197 SKYLARK CO.,LTD 3770 ZAPPALLAS,INC. 3232 Mie Kotsu Group Holdings,Inc. 4007 Nippon Kasei Chemical Company Limited 3252 Nippon Commercial Development Co.,Ltd. 4097 KOATSU GAS KOGYO CO.,LTD. 3276 Japan Property Management Center Co.,Ltd. 4098 Titan Kogyo Kabushiki Kaisha 3385 YAKUODO.Co.,Ltd. 4275 Carlit Holdings Co.,Ltd. 3553 KYOWA LEATHER CLOTH CO.,LTD. 4295 Faith, Inc. 3649 FINDEX Inc. 4326 INTAGE HOLDINGS Inc. 3660 istyle Inc. 4344 SOURCENEXT CORPORATION 3681 V-cube,Inc. 4671 FALCO HOLDINGS Co.,Ltd. 3751 Japan Asia Group Limited 4779 SOFTBRAIN Co.,Ltd. 3844 COMTURE CORPORATION 4801 CENTRAL SPORTS Co.,LTD. -

Kyowa Hakko Kirin Annual Report 2009/12 All
BIOTECHNOLOGY FOR GLOBAL HEALTH Kyowa Hakko KirinKyowa Annual Report 2009/12 Kyowa Hakko Kirin Annual Report 2009/12 Transitional nine-month period ended December 31, 2009 AboUT KyoWA Hakko KIRin Kyowa Hakko Kirin Co., Ltd., was inaugurated in October 2008 as an R&D- based company with special strengths in biotechnology, following the integration of Kirin Pharma Company, Limited, of the Kirin Group, and Kyowa Hakko Kogyo Co., Ltd. The Company is dedicated to the creation of new value in the life sciences, especially in its core business segments of Pharmaceuti- cals and Bio-Chemicals, and strives to contribute to the health and well-being of people around the world. We are seeking new heights by aggressively pro- moting our proprietary technologies in each business domain. In Pharmaceuticals operations, the Company has actively engaged in the R&D, production, and sale of pharmaceuticals that address medical needs in such areas as renal anemia, cancer, allergies, and hypertension. Utilizing leading-edge biotechnologies, particularly antibody technologies, we are aim- ing to be a global specialty pharmaceutical company that creates innovative pharmaceuticals. Bio-Chemicals operations are centered on Kyowa Hakko Bio Co., Ltd., which was established as a separate company at the same time as the inauguration of Kyowa Hakko Kirin and is a global leader in fermented bulk products, such as amino acids, nucleic acids, and related compounds. In Chemicals operations, the Company is expanding lineups of specialty chemicals that contribute to environmental -

Survey of Integrated Reports in Japan 2018
Survey of Integrated Reports in Japan 2018 Integrated Reporting Center of Excellence KPMG in Japan March 2019 home.kpmg/jp Message from global thought leaders In this, the fifth year this survey report has been issued, KPMG has solicited the observations of thought leaders on corporate reporting. Japanese businesses pride themselves on a longer-term focus compared to the rest of the world, where prioritizing short-term gains has too often become the norm. Focusing on long-term value creation and taking into account all of the resources an organization uses is the sustainable, profitable, and proven way to manage a business. Many Japanese businesses are just beginning to implement integrated thinking and reporting. The findings in this report show that senior management must take ownership to spread integrated thinking in their businesses – not just in accounting but in strategy, operations, marketing, and the rest of the company as well. This report makes me optimistic and excited for Japanese business leaders as they strive to think, act, and communicate in an integrated and sustainable way. Dominic Barton ― International Integrated Reporting Council, Chairman Integrated Reporting improves communication between companies and investors and where most effective, sets the stage for enhanced corporate value creation over the mid to long-term. Investors desire integrated reports which provide comprehensive information disclosure useful for making investment decisions. The International Corporate Governance Network (ICGN) has encouraged integrated reporting for many years. In 2015, ICGN s Disclosure and Transparency Committee ’ released Guidance on Integrated Business Reporting. The Guidance amplifies that strategic decisions should be based on factors that are broader than those reflected in the financial statements.