Seabird Ecology & Conservation (MARS4040) Quiz #3: Adaptations / Ecomorphology/ Lectures 1 Student Name: KEY This 30-Minute
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Southwest Pacific Islands: Samoa, Fiji, Vanuatu & New Caledonia Trip Report 11Th to 31St July 2015
Southwest Pacific Islands: Samoa, Fiji, Vanuatu & New Caledonia Trip Report 11th to 31st July 2015 Orange Fruit Dove by K. David Bishop Trip Report - RBT Southwest Pacific Islands 2015 2 Tour Leaders: K. David Bishop and David Hoddinott Trip Report compiled by Tour Leader: K. David Bishop Tour Summary Rockjumper’s inaugural tour of the islands of the Southwest Pacific kicked off in style with dinner at the Stamford Airport Hotel in Sydney, Australia. The following morning we were soon winging our way north and eastwards to the ancient Gondwanaland of New Caledonia. Upon arrival we then drove south along a road more reminiscent of Europe, passing through lush farmlands seemingly devoid of indigenous birds. Happily this was soon rectified; after settling into our Noumea hotel and a delicious luncheon, we set off to explore a small nature reserve established around an important patch of scrub and mangroves. Here we quickly cottoned on to our first endemic, the rather underwhelming Grey-eared Honeyeater, together with Nankeen Night Herons, a migrant Sacred Kingfisher, White-bellied Woodswallow, Fantailed Gerygone and the resident form of Rufous Whistler. As we were to discover throughout this tour, in areas of less than pristine habitat we encountered several Grey-eared Honeyeater by David Hoddinott introduced species including Common Waxbill. And so began a series of early starts which were to typify this tour, though today everyone was up with added alacrity as we were heading to the globally important Rivierre Bleu Reserve and the haunt of the incomparable Kagu. We drove 1.3 hours to the reserve, passing through a stark landscape before arriving at the appointed time to meet my friend Jean-Marc, the reserve’s ornithologist and senior ranger. -

Order CHARADRIIFORMES: Waders, Gulls and Terns Suborder LARI
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 191, 223, 230 & 232-233. Order CHARADRIIFORMES: Waders, Gulls and Terns The family sequence of Christidis & Boles (1994), who adopted that of Sibley et al. (1988) and Sibley & Monroe (1990), is followed here. Suborder LARI: Skuas, Gulls, Terns and Skimmers Condon (1975) and Checklist Committee (1990) recognised three subfamilies within the Laridae (Larinae, Sterninae and Megalopterinae) but this division has not been widely adopted. We follow Gochfeld & Burger (1996) in recognising gulls in one family (Laridae) and terns and noddies in another (Sternidae). The sequence of species for Stercorariidae and Laridae follows Peters (1934) and for Sternidae follows Bridge et al. (2005). Family STERNIDAE Bonaparte: Terns and Noddies Sterninae Bonaparte, 1838: Geogr. Comp. List. Birds: 61 – Type genus Sterna Linnaeus, 1758. Most recommendations from a new study of tern and noddy relationships, based on mtDNA (Bridge et al. 2005), have already been adopted by the Taxonomic Subcommittee of the British Ornithologists’ Union Records Committee (Sangster et al. 2005) and the American Ornithologists’ Union Committee on Classification and Nomenclature (Banks, R.C. et al. 2006). This follows many years of disagreement about the generic classification of terns for which 3–12 genera have recently been used (see Bridge et al. 2005). The genera and their sequence recommended by Bridge et al. -

A Checklist of the Birds of Micronesia
A Checklist of the Birds of Micronesia ROBERT P. OWEN Chief Conservationist, Trust Territory of the Pacific Islands Koror, Palau, Caroline Islands 96940 Abstract.-This paper lists 191 species of birds found in Micronesia based on publications on collections and acceptable sight records. The last published checklist of Micronesian birds was published by Baker (1951) and lists 151 species. This present checklist records the English names, scientific names, and geographic localities within Micronesia in which the different species are found, and also gives status information on the birds such as whether resident, migrant or vagrant, and whether introduced, endangered or extinct. The most comprehensive publication on Micronesian birds is that produced by Baker (1951). That publication was based on field work done by Rollin H. Baker and other U. S. military biologists carried out in Micronesia in 1945 and a review of all publications on Micronesian ornithology prior to that date, and up to 1948. In the intervening 29 years, a good many ornithologists and other biologists have traveled or resided in Micronesia for various periods of time and have published accounts of birds seen or collected in Micronesia. Baker (1951) listed 151 full species of birds in Micronesia. The present checklist (Table 1) contains 191 full species. Thus 40 bird species have been added to the list of birds known from Micronesia from published records. Approximately one fourth of the bird species listed in this checklist are based on published sight records. Great care has been used in evaluating published sight records and several have been rejected for one reason or another for inclusion in this publication. -

Threats to Seabirds: a Global Assessment 2 3 4 Authors: Maria P
1 Threats to seabirds: a global assessment 2 3 4 Authors: Maria P. Dias1*, Rob Martin1, Elizabeth J. Pearmain1, Ian J. Burfield1, Cleo Small2, Richard A. 5 Phillips3, Oliver Yates4, Ben Lascelles1, Pablo Garcia Borboroglu5, John P. Croxall1 6 7 8 Affiliations: 9 1 - BirdLife International. The David Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK 10 2 - BirdLife International Marine Programme, RSPB, The Lodge, Sandy, SG19 2DL 11 3 – British Antarctic Survey. Natural Environment Research Council, High Cross, Madingley Road, 12 Cambridge CB3 0ET, UK 13 4 – Centre for the Environment, Fishery and Aquaculture Science, Pakefield Road, Lowestoft, NR33, UK 14 5 - Global Penguin Society, University of Washington and CONICET Argentina. Puerto Madryn U9120, 15 Chubut, Argentina 16 * Corresponding author: Maria Dias, [email protected]. BirdLife International. The David 17 Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK. Phone: +44 (0)1223 747540 18 19 20 Acknowledgements 21 We are very grateful to Bartek Arendarczyk, Sophie Bennett, Ricky Hibble, Eleanor Miller and Amy 22 Palmer-Newton for assisting with the bibliographic review. We thank Rachael Alderman, Pep Arcos, 23 Jonathon Barrington, Igor Debski, Peter Hodum, Gustavo Jimenez, Jeff Mangel, Ken Morgan, Paul Sagar, 24 Peter Ryan, and other members of the ACAP PaCSWG, and the members of IUCN SSC Penguin Specialist 25 Group (Alejandro Simeone, Andre Chiaradia, Barbara Wienecke, Charles-André Bost, Lauren Waller, Phil 26 Trathan, Philip Seddon, Susie Ellis, Tom Schneider and Dee Boersma) for reviewing threats to selected 27 species. We thank also Andy Symes, Rocio Moreno, Stuart Butchart, Paul Donald, Rory Crawford, 28 Tammy Davies, Ana Carneiro and Tris Allinson for fruitful discussions and helpful comments on earlier 29 versions of the manuscript. -

Natural History Guide to American Samoa
NATURAL HISTORY GUIDE TO AMERICAN SAMOA rd 3 Edition NATURAL HISTORY GUIDE This Guide may be available at: www.nps.gov/npsa Support was provided by: National Park of American Samoa Department of Marine & Wildlife Resources American Samoa Community College Sport Fish & Wildlife Restoration Acts American Samoa Department of Commerce Pacific Cooperative Studies Unit, University of Hawaii American Samoa Coral Reef Advisory Group National Oceanic and Atmospheric Administration Natural History is the study of all living things and their environment. Cover: Ofu Island (with Olosega in foreground). NATURAL HISTORY GUIDE NATURAL HISTORY GUIDE TO AMERICAN SAMOA 3rd Edition P. Craig Editor 2009 National Park of American Samoa Department Marine and Wildlife Resources Pago Pago, American Samoa 96799 Box 3730, Pago Pago, American Samoa American Samoa Community College Community and Natural Resources Division Box 5319, Pago Pago, American Samoa NATURAL HISTORY GUIDE Preface & Acknowledgments This booklet is the collected writings of 30 authors whose first-hand knowledge of American Samoan resources is a distinguishing feature of the articles. Their contributions are greatly appreciated. Tavita Togia deserves special recognition as contributing photographer. He generously provided over 50 exceptional photos. Dick Watling granted permission to reproduce the excellent illustrations from his books “Birds of Fiji, Tonga and Samoa” and “Birds of Fiji and Western Polynesia” (Pacificbirds.com). NOAA websites were a source of remarkable imagery. Other individuals, organizations, and publishers kindly allowed their illustrations to be reprinted in this volume; their credits are listed in Appendix 3. Matt Le'i (Program Director, OCIA, DOE), Joshua Seamon (DMWR), Taito Faleselau Tuilagi (NPS), Larry Basch (NPS), Tavita Togia (NPS), Rise Hart (RCUH) and many others provided assistance or suggestions throughout the text. -
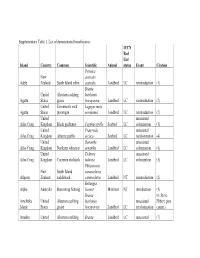
Supplementary Table 1. List of Demonstrated Beneficiaries
Supplementary Table 1. List of demonstrated beneficiaries. IUCN Red List Island Country Common Scientific Animal status Event Citation Petroica New australis Adele Zealand South Island robin australis Landbird LC reintroduction (1) Branta United Aleutian cackling hutchinsii Agattu States goose leucopareia Landbird LC reintroduction (2) United Evermann's rock Lagopus muta Agattu States ptarmigan evermanni Landbird LC reintroduction (2) United unassisted Ailsa Craig Kingdom Black guillemot Cepphus grylle Seabird LC colonization (3) United Fratercula unassisted Ailsa Craig Kingdom Atlantic puffin arctica Seabird LC recolonization (4) United Oenanthe unassisted Ailsa Craig Kingdom Northern wheatear oenanthe Landbird LC colonization (4) United Tadorna unassisted Ailsa Craig Kingdom Common shelduck tadorna Landbird LC colonization (3) Philesturnus New South Island carunculatus Allports Zealand saddleback carunculatus Landbird NT reintroduction (2) Bettongia Alpha Australia Burrowing bettong lesueur Mammal NT introduction (5) Branta (6; Steve Amchitka United Aleutian cackling hutchinsii unassisted Ebbert, pers. Island States goose leucopareia Landbird LC recolonization comm.) Amukta United Aleutian cackling Branta Landbird LC unassisted (7) IUCN Red List Island Country Common Scientific Animal status Event Citation States goose hutchinsii recolonization leucopareia Sally Amy Poncet, Island/Outer United Cinclodes unassisted unpublished Knob Kingdom Tussacbird antarcticus Landbird LC recolonization data Sally Amy Poncet, Island/Outer United unpublished -

Annotated List of Birds Observed on Christmas Island, October to December 1953
Annotated List of Birds Observed on Christmas Island, October to December 1953 JOSEPH E. KINd IN OCTOBER 1953, the Pacific Oceanic Fishery low, averaging about 10 feet or less in eleva Investigations of the U. S. Fish and Wildlife tion, except along the southern shore of the Service landed a small field party (the author Bay of Wrecks where there are scattered sand and Mr. Richard Shomura) on Christmas Is dunes which may reach 40 feet in height. land for the purpose of installing weather The rainfall is quite variable but averages instruments and sea thermographs, and to 25 to 35 inches a year (Bryan, 1942). Frag conduct a tuna bait-fish survey of the lagoon. mentary records show an annual range from We were on the island from October 23 to 10 inches to as much as 100 inches. The air December 9, 1953, and although our time temperature varies from a minimum of 68° was generally occupied with the duties men or 70° F. to a maximum of 106° F. (Went tioned above, we had many opportunities to worth, 1931). observe the interesting bird life of the island. Christophersen (1927) records 24 species As Christmas and other islands of the Line of vascular plants as growing naturally on Island group (Fanning, Washington, and Christmas Island. These include Tournefortia Palmyra) are rarely visited by ornithologists, argentea trees, clumps ofScaevola frutescens and we thought it worthwhile to report our ob other low shrubs, grasses, and herbs. Al servations. though all the coconut palms now seen on the The author is indebted to Dr. -

The Black Noddy Anous Minutus: a New Breeding Species for Chile
Marín et al.: First record of breeding Black Noddies in Chile 79 THE BLACK NODDY ANOUS MINUTUS: A NEW BREEDING SPECIES FOR CHILE MANUEL MARÍN1,2, RODRIGO GONZÁLEZ3 & SERGIO TRUCCO4 1Natural History Museum of Los Angeles County, Section of Ornithology, 900 Exposition Boulevard, Los Angeles, California 90007, USA 2Current address: Casilla 15 Melipilla, Chile ([email protected]) 3Santa María 7178, Vitacura, Santiago, Chile 4Jacarepagua 10156, Vitacura, Santiago, Chile Received 29 June 2020, accepted 25 November 2020 ABSTRACT MARÍN, M., GONZÁLEZ, R. & TRUCCO, S. 2021. The Black Noddy Anous minutus: A new breeding species for Chile. Marine Ornithology 49: 79–82. A small breeding population of Black Noddy Anous minutus was encountered on San Ambrosio Island in the Desventuradas Archipelago in the southeast Pacific off Chile. On 11 December 2019, we found eight Black Noddy nests among 50–60 nests of Brown Noddies A. stolidus. Black Noddy nests were placed on the ground with little to no nesting material in the interior of the island, which is an unusual nest type and placement for this species. All nests were at different stages, from recently hatched to recently fledged nestlings. Black Noddies usually nest in trees or bushes, but the vegetation on San Ambrosio had been largely extirpated, raising the possibility that this small population may have been larger prior to habitat loss. This is the first published documented record of Black Noddies in Chile and is the southeasternmost breeding population of this species in the Pacific. Key words: Black Noddy, Anous minutus, breeding, San Ambrosio Island, eastern South Pacific, Chile The Black Noddy Anous minutus is a widely distributed pantropical published Jehl (1973); and observations made in June and December colonial species that nests in the subtropical and tropical zones of 2001, and in March 2003, both published in Aguirre et al. -

Important Bird Areas of Samoa
IMPORTANT BIRD AREAS OF SAMOA Prepared by: Toeolesulusulu Cedric Schuster Birdlife International in partnership with Conservation International, O Le Siosiomaga Society Incorporated and the Ministry of Natural Resources and Environment ACKNOWLEDGEMENT We wish to acknowledge several people who contributed tremendous effort and time in the preparation of this report. Firstly we extend a special appreciation for James Atherton of Conservation International for the numerous contribution to the report which includes the preparation of all the maps, the Samoa Review of Important Bird Areas report he wrote with Toni Tipamaa of MNRE which provided the majority of the information used in this report, and additional time spent on the review and commenting on the report. We also wish to acknowledge with appreciation all the members of the Steering Committee for the this project which includes, Fiu Mataese Elisara (Director of OLSSI); Toni Tipamaa (ACEO MNRE); Tepa Suaesi (SPREP) and finally Steve Cranwell and James Millett of Birdlife Pacific for all the time and effort put in to review and comment on the report as well as organizing and presenting at the national and community workshops. 2 | P a g e Contents ACKNOWLEDGEMENT ............................................................................................................................2 BACKGROUND ........................................................................................................................................4 SAMOA...................................................................................................................................................4 -

Expansion of the U.S. Pacific Remote Islands Marine National Monument ! the Largest Ocean Legacy on Earth ! ! May 20, 2014
Expansion of the U.S. Pacific Remote Islands Marine National Monument ! The largest ocean legacy on Earth ! ! May 20, 2014 Report to the United States government prepared by: Dr. Enric Sala - NaHonal Geographic Society, Washington, DC Dr. Lance Morgan - Marine ConservaHon InsHtute, Sonoma, CA Dr. EllioN Norse - Marine ConservaHon InsHtute, Redmond, WA Dr. Alan Friedlander, University of Hawaii, Honolulu, HI Report on expansion of Pacific Remote Islands Marine National Monument – May 20, 2014 Expansion of the U.S. Pacific Remote Islands Marine National Monument EXECUTIVE SUMMARY Presidential Leadership and Legacy President Obama has the authority and opportunity to leave the largest ocean conservation legacy in history by extending the existing U.S. Pacific Remote Islands Marine National Monument from the current 50 nautical miles around the seven U.S. Pacific Remote Islands out to the full extent of its 200-nautical mile Exclusive Economic Zone (EEZ) – an increase of 1.8 million square km of new protected area (increase 10 times the current 225,000 square km Monument). That action would create the largest protected area on Earth (an expanded Monument over 2 million square km) – and include some of the world’s most pristine deep sea and open ocean ecosystems, with unique and global biodiversity value1. These pristine national treasures would receive full protection, meaning no extractive activities such as mining, drilling, and fishing would be allowed. The new National Monument alone would protect 18 percent of the United States EEZ, and it would double the area of the ocean that is currently fully protected globally2. This would make the United States the undisputable world leader in ocean conservation, and set a record in conservation that is unlikely to be matched again in the U.S. -

A Global Overview of Wetland and Marine Protected Areas on the World Heritage List
A GLOBAL OVERVIEW OF WETLAND AND MARINE PROTECTED AREAS ON THE WORLD HERITAGE LIST A Contribution to the Global Theme Study of World Heritage Natural Sites Prepared by Jim Thorsell, Renée Ferster Levy and Todd Sigaty Natural Heritage Programme, IUCN Gland, Switzerland in collaboration with The World Conservation Monitoring Centre. September 1997 Working Paper 1: Earth’s Geological History - A Contextual Framework Assessment of World Heritage Fossil Site Nominations Working Paper 2: A Global Overview of Wetland and Marine Protected Areas on the World Heritage List Working Paper 3: A Global Overview of Forest Protected Areas on the World Heritage List Further volumes (in preparation) on biodiversity, mountains, deserts and grasslands, and geological features. TABLE OF CONTENTS PAGE I. Executive Summary (e/f) II. Introduction 1 III. Tables & Figures Table 1. Natural World Heritage sites with primary wetland and marine values 11 Table 2. Natural World Heritage sites with secondary wetland and marine values 12 Table 3. Natural World Heritage sites inscribed primarily for their freshwater wetland values 13 Table 4. Additional natural World Heritage sites with significant freshwater wetland values 14 Table 5. Natural World Heritage sites with a coastal/marine component 15 Table 6. Natural World Heritage sites containing mangroves 16 Table 7. Island natural World Heritage sites 17 Table 8. Natural World Heritage sites containing coral reef 18 Table 9. Natural World Heritage sites with subterranean rivers and lakes 18 Table 10. Natural World Heritage sites with wetland and marine values included in the List of World Heritage in Danger 19 Table 11. Regions with significant wetland and marine values that contain areas which may merit consideration for World Heritage nomination 20 Figure 1. -

Redalyc.Seabirds of Easter Island, Salas Y Gómez Island and Desventuradas Islands, Southeastern Pacific Ocean
Latin American Journal of Aquatic Research E-ISSN: 0718-560X [email protected] Pontificia Universidad Católica de Valparaíso Chile Flores, Marcelo A.; Schlatter, Roberto P.; Hucke-Gaete, Rodrigo Seabirds of Easter Island, Salas y Gómez Island and Desventuradas Islands, southeastern Pacific Ocean Latin American Journal of Aquatic Research, vol. 42, núm. 4, octubre, 2014, pp. 752-759 Pontificia Universidad Católica de Valparaíso Valparaiso, Chile Available in: http://www.redalyc.org/articulo.oa?id=175032366006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Lat. Am. J. Aquat. Res., 42(4): 752-759, 2014 Seabirds of Chilean oceanic islands 752 1 “Oceanography and Marine Resources of Oceanic Islands of Southeastern Pacific” M. Fernández & S. Hormazábal (Guest Editors) DOI: 10.3856/vol42-issue4-fulltext-6 Review Seabirds of Easter Island, Salas y Gómez Island and Desventuradas Islands, southeastern Pacific Ocean Marcelo A. Flores1, Roberto P. Schlatter2 & Rodrigo Hucke-Gaete2,3 1Departamento de Ecología y Biodiversidad, Facultad de Ecología y Recursos Naturales Universidad Andres Bello, República 470-Piso 3, Santiago, Chile 2Instituto de Ciencias Marinas y Limnológicas (ICML), Universidad Austral de Chile P.O. Box 567, Valdivia, Chile 3Centro Ballena Azul, c/o ICML, Universidad Austral de Chile, P.O. Box 567, Valdivia, Chile ABSTRACT. We reviewed available information on seabirds inhabiting Easter Island, Salas y Gómez Island and Desventuradas Islands and their adjacent waters through an analysis of published and grey literature.