321-326 First Records and Abundance of Margay Leopardus Wiedii From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
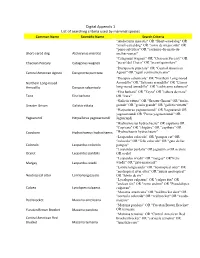
Digital Appendix 1 List of Searching Criteria Used by Mammal Species
Digital Appendix 1 List of searching criteria used by mammal species Common Name Scientific Name Search Criteria "Atelocynus microtis" OR "Short-eared dog" OR "small-eared dog" OR "zorro de oreja corta" OR "perro selvático" OR "cachorro-do-mato-de- Short-eared dog Atelocynus microtis orelhas-curtas" "Catagonus wagneri" OR "Chacoan Peccary" OR Chacoan Peccary Catagonus wagneri "pecarí del Chaco" OR "pecarí quimilero" “Dasyprocta punctate” OR "Central American Central American Agouti Dasyprocta punctata Agouti" OR "agutí centroamericano" “Dasypus sabanicola” OR "Northern Long-nosed Northern Long-nosed Armadillo" OR "Savanna armadillo" OR "Llanos Armadillo Dasypus sabanicola long-nosed armadillo" OR "cachicamo sabanero" “Eira barbara” OR "Tayra" OR "cabeza de mate" Taira Eira barbara OR "irara" “Galictis vittata” OR "Greater Grison" OR "furão- Greater Grison Galictis vittata grande" OR "grisón grande" OR "galictis vittatta" “Herpailurus yagouaroundi” OR Yaguarundi OR yagouaroundi OR "Puma yagouaroundi" OR Yaguarundi Herpailurus yagouaroundi Jaguarundi "Hydrochoerus hydrochaeris" OR capybara OR "Capivara" OR "chigüire" OR "capibara" OR Capybara Hydrochoerus hydrochaeris "Hydrochaeris hydrochaeris" “Leopardus colocolo” OR "pampas cat" OR "colocolo" OR "felis colocolo" OR "gato de las Colocolo Leopardus colocolo pampas" "Leopardus pardalis" OR jaguatirica OR ocelote Ocelot Leopardus pardalis OR ocelot “Leopardus wiedii” OR "margay" OR"Felis Margay Leopardus wiedii wiedii" OR "gato-maracajá" “Lontra longicaudis” OR "neotropical otter" OR "neotropical -

Galictis Cuja Molina, 1782) As Host of Dioctophyme Renale Goeze, 1782 Furão Pequeno (Galictis Cuja Molina, 1782) Como Hospedeiro De Dioctophyme Renale Goeze, 1782
ANIMAL PARASITOLOGY / SCIENTIFIC COMMUNICATION DOI: 10.1590/1808-1657000312016 Lesser Grison (Galictis cuja Molina, 1782) as host of Dioctophyme renale Goeze, 1782 Furão Pequeno (Galictis cuja Molina, 1782) como hospedeiro de Dioctophyme renale Goeze, 1782 Daniela Pedrassani1*, Mayana Worm1, Jéssica Drechmer1, Margareth Cristina Iazzetti Santos1 ABSTRACT: The Dioctophyme renale is a helminth parasite RESUMO: O Dioctophyme renale é um helminto parasita renal of the kidney usually seen in domestic and wild carnivores and observado normalmente em carnívoros domésticos e silvestres e rarely in human beings. This is a report about the parasitism excepcionalmente em seres humanos. Relata-se o parasitismo por D. of D. renale found in the kidney of two roadkill lesser grisons renale em rim de dois furões pequenos (Galictis cuja) encontrados (Galictis cuja) in the North of the state of Santa Catarina, mortos por atropelamento no Norte do estado de Santa Catarina, Brazil. The report of this parasitism in this species is important Brasil. Relatar esse parasitismo nessa espécie é importante, para to complement the records about this native carnivore as a que se possam somar dados relativos a participação deste carnívoro contributor in the epidemiologic chain while host/disseminator nativo na cadeia epidemiológica como hospedeiro/ veiculador desse of this helminth with zoonotic potential. helminto com potencial zoonótico. KEYWORDS: Dioctophyma; wild animal; mustelids; roadkill; PALAVRAS-CHAVE: Dioctophyma; animal silvestre; mustelí- kidney parasitism. deo; atropelamento em rodovia; parasitismo renal. 1Universidade do Contestado (UnC) – Canoinhas (SC), Brazil. *Corresponding author: [email protected] Received on: 04/22/2016. Accepted on: 09/12/2017 Arq. Inst. Biol., v.84, 1-4, e0312016, 2017 1 D. -

Grow, Forest, Grow a Toolkit for Investors Concerned About Deforestation – Focus on Brazil and the Protein Industry
Europe Equity Research 22 January 2021 Grow, Forest, Grow A toolkit for investors concerned about deforestation – Focus on Brazil and the Protein industry This reports explores the sustainability impacts and the financial risks related ESG & Sustainability research to deforestation and forest degradation, using Brazil as our key example. Based AC on the takeaways from JPM LatAm ESG “Protein” seminars, we discuss the key Jean-Xavier Hecker (33-1) 4015 4472 commitments taken by JBS, Marfrig, Minerva and BRF, and outline where we [email protected] see areas for improvement and opportunities related to regenerative agriculture. J.P. Morgan Securities plc Deforestation is an emerging theme for ESG investors. To date, investors Hugo Dubourg that include deforestation in their ESG approach are mostly driven by the (33-1) 4015 4471 [email protected] "sustainable materiality” of the theme’ (climate & biodiversity). As ESG AUMs J.P. Morgan Securities plc keep growing, we believe that a company which has significant exposure to FRCs and ignores related risks could suffer from a proportionate discount in LatAm Food & Beverages and Agribusiness valuation. We see this as stemming from several different ESG strategies: AC 1) Discount; 2) Outflows and 3) Missed opportunities. Over the long term, Lucas Ferreira these strategies are likely to impact valuations, resulting in a material discount (55-11) 4950-3629 [email protected] vs. a company’s historical average. Bloomberg JPMA FERREIRA <GO> Banco J.P. Morgan S.A. For countries such as Brazil, which holds 12% of the world’s total forests, deforestation represents a major issue, which has gained notoriety since 2019. -

The Impact of Seasonal Climate on New Case Detection Rate of Leprosy in Brazil (2008–2012)
Lepr Rev (2017) 88, 533–542 The impact of seasonal climate on new case detection rate of leprosy in Brazil (2008–2012) ALINE CRISTINA ARAU´ JO ALCAˆ NTARA ROCHA*, WASHINGTON LEITE JUNGER**, WESLEY JONATAR ALVES DA CRUZ* & ELIANE IGNOTTI* *University of the State of Mato Grosso - UNEMAT, Rua dos Tuiuiu´s, 660 Vila Mariana, Ca´ceres-MT-Brasil, CEP. 78200-000 **University of the State of Rio de Janeiro - UERJ, Institute of Social Medicine, Rio de Janeiro - RJ, Brasil Accepted for publication 2 August 2017 Summary Objective: To examine the impact of climatic seasonality in the new case detection rate of leprosy in Brazil according to geographical regions, climates and biomes over a 5-year period, 2008–2012. Methods: We conducted an ecological study of the monthly new case detection rate of leprosy in spatial aggregation of Brazilian geographical regions, climates and biomes, applying a linear regression models with Poisson function to estimate seasonal rates using January as the reference month. Results: Monthly seasonal patterns of leprosy detection rates were recorded between different geographic regions, biomes and climates, with a predominance of increases in the autumn, in the months of March and May, and in winter in the month of August. Conclusions: The detection rate of leprosy in Brazil has a seasonal pattern with specific variations between geographical regions, climates, and biomes. The highest peaks in the detection rates were observed in May (autumn) and in August (winter). In addition to the supply and accessibility of healthcare -

Genetic Verification of Multiple Paternity in Two Free-Ranging Isolated Populations of African Wild Dogs (Lycaon Pictus)
University of Pretoria etd – Moueix, C H M (2006) Genetic verification of multiple paternity in two free-ranging isolated populations of African wild dogs (Lycaon pictus) by Charlotte Moueix Submitted in partial fulfilment of the requirements for the degree of Master of Science Department of Production Animal Studies Faculty of Veterinary Science University of Pretoria Onderstepoort Supervisor Prof HJ Bertschinger Co-supervisors Dr CK Harper Dr ML Schulman August 2006 University of Pretoria etd – Moueix, C H M (2006) DECLARATION I, Charlotte Moueix, do hereby declare that the research presented in this dissertation, was conceived and executed by myself, and apart from the normal guidance from my supervisor, I have received no assistance. Neither the substance, nor any part of this dissertation has been submitted in the past, or is to be submitted for a degree at this University or any other University. This dissertation is presented in partial fulfilment of the requirements for the degree MSc in Production Animal Studies. I hereby grant the University of Pretoria free license to reproduce this dissertation in part or as whole, for the purpose of research or continuing education. Signed ……………………………. Charlotte Moueix Date ………………………………. ii University of Pretoria etd – Moueix, C H M (2006) ACKNOWLEDGEMENTS I have been lucky to have people at my side whose support, encouragement and wisdom made all the difference. The following people and organisations contributed to the completion of this work, either materially or through their expertise or moral support: Prof. Henk Bertschinger, for trusting me with this project and making it possible. Dr. Martin Schulman, for his expert advice. -

Changes in Tree Community Diversity and Composition on Climatic and Geographic Gradients
RESEARCH ARTICLE The Brazilian freshwater wetscape: Changes in tree community diversity and composition on climatic and geographic gradients Florian Wittmann1,2, MaÂrcia C. M. Marques3, Geraldo Damasceno JuÂnior4, Jean Carlos Budke5, Maria T. F. Piedade2, Astrid de Oliveira Wittmann6, Juan Carlos Montero7, Rafael L. de Assis2,8, NataÂlia Targhetta2, Pia Parolin9, Wolfgang J. Junk10, J. Ethan Householder1,11* a1111111111 1 Department of Floodplain Ecology, Institute of Geography and Geoecology, Karlsruhe Institute for Technology, Karlsruhe, Germany, 2 MAUA Working Group, Instituto Nacional de Pesquisas da AmazoÃnia, a1111111111 Manaus, Amazonas, Brazil, 3 Universidade Federal do ParanaÂ, Curitiba, ParanaÂ, Brazil, 4 Universidade a1111111111 Federal do Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brazil, 5 Universidade Regional a1111111111 Integrada do Alto Uruguai e das Missões, Erechim, Rio Grande do Sul, Brazil, 6 Universidade Federal do a1111111111 Amazonas, Manaus, Amazonas, Brazil, 7 ConfederacioÂn de Pueblos IndõÂgenas de Bolivia, Gobernanza de Recursos Naturales, Santa Cruz de la Sierra, Santa Cruz, Bolivia, 8 Norwegian University of Life Sciences, Ås, Akershus, Norway, 9 University of Hamburg, Biocentre Klein Flottbek, Department of Plant Diversity, Hamburg, Germany, 10 Instituto Nacional de AÂ reas UÂ midas, CuiabaÂ, Mato Grosso, Brazil, 11 Botanical Research Institute of Texas, Fort Worth, Texas, United States of America OPEN ACCESS * [email protected] Citation: Wittmann F, Marques MCM, Damasceno JuÂnior G, Budke JC, Piedade MTF, de Oliveira Wittmann A, et al. (2017) The Brazilian freshwater Abstract wetscape: Changes in tree community diversity and composition on climatic and geographic Wetlands harbor an important compliment of regional plant diversity, but in many regions gradients. -

I METHODS of NICHE PARTITIONING BETWEEN
METHODS OF NICHE PARTITIONING BETWEEN ECUADORIAN CARNIVORES AND HABITAT PREFERENCE OF THE MARGAY ( LEOPARDUS WIEDII ) Anne-Marie C. Hodge A Thesis Submitted to the University of North Carolina Wilmington in Partial Fulfillment for the Requirements for the Degree of Master of Science Department of Biology and Marine Biology University of North Carolina Wilmington 2012 Approved By: Advisory Committee Travis Knowles Steven Emslie Marcel van Tuinen Brian Arbogast Chair Accepted by Dean, Graduate School i TABLE OF CONTENTS ABSTRACT ................................................................................................................................... iv DEDICATION .................................................................................................................................v LIST OF TABLES ......................................................................................................................... vi LIST OF FIGURES ...................................................................................................................... vii CHAPTER 1: MARGAY ACTIVITY PATTERNS AND DENSITY............................................1 Introduction ..........................................................................................................................1 Methods................................................................................................................................5 Study Location .........................................................................................................5 -

New Record for Bush Dog in Amapá State, Eastern Brazilian Amazonia
Michalski et al. Bush dogs in Eastern Brazilian Amazonia Copyright © 2015 by the IUCN/SSC Canid Specialist Group. ISSN 1478-2677 Distribution Update New record for bush dog in Amapá State, Eastern Brazilian Amazonia Lincoln J. Michalski*1,2, Tadeu G. de Oliveira3,4 and Fernanda Michalski1,2,4,5 1 Instituto Nacional de Pesquisas da Amazônia, Av. André Araújo, 2936, 69060-001 - Manaus, AM, Brazil. Email: [email protected] 2 Laboratório de Ecologia e Conservação de Vertebrados, Universidade Federal do Amapá, Rod. Juscelino Kubitscheck, km 02, 68903-419 - Macapá, AP - Brazil. 3 Departamento de Biologia, Universidade Estadual do Maranhão, Rua das Quaresmeiras, Qd-08, N°. 14, 65076-270 - São Luís, MA, Brazil. 4 Instituto Pró-Carnívoros, C.P. 10, 12940-970 - Atibaia, SP - Brazil. 5 Programa de Pós-Graduação em Biodiversidade Tropical, Universidade Federal do Amapá, Rod. Juscelino Kubitscheck, Km 02, 68903-419 - Macapá, AP - Brazil. * Correspondence author Keywords: Amapá National Forest, Amazon Forest, camera trap, geographic distribution, Speothos venaticus. Abstract Bush dogs are considered one of the lesser-known canids of South America. We report an update on their distribution in the north region of Brazil. Three bush dogs were filmed by a camera trap in Amapá Na- tional Forest, Eastern Brazilian Amazonia. The record occurred during data collection for a long-term study of medium and large vertebrates. On 28 March 2014 at 22:13h, three bush dogs passed in front of the camera. This record increases knowledge on the distribution of the species. Despite its large geographic range, bush dogs Speothos venaticus areas, bush dogs are mostly associated with well preserved areas (Lund, 1842) have been proven to be extremely difficult to locate in (Oliveira 2009) or in large forest fragments (Carretero-Pinzón 2013), the wild (DeMatteo and Loiselle 2008, DeMatteo et al. -

Cerdocyon Thous Compiled by the Amazonian Canids Working Group – 01/2021
Literature: Cerdocyon thous Compiled by the Amazonian Canids Working Group – 01/2021 Abra FD, da Costa Canena, Garbino GST, & Medici EM. 2020. Use of unfenced highway underpasses by lowland tapirs and other medium and large mammals in central-western Brazil. Perspectives in Ecology and Conservation 18(4):247-256. Aguirre LF, Tarifa T, Wallace RB, Bernal N, Siles L, Aliaga-Rossel E & Salazar-Bravo J. 2019. Lista actualizada y comentada de los mamíferos de Bolivia. Ecología en Bolivia 54(2):107-147. Almeida, AP, Souza, TD, Marcili, A, & Labruna, MB. 2013. Novel Ehrlichia and Hepatozoon agents infecting the crab-eating fox (Cerdocyon thous) in southeastern Brazil. Journal of Medical Entomology 50(3):640-646. Aya-Cuero CA, Mosquera-Guerra F, Esquivel DA, Trujillo F, & Brooks D. 2019. Medium and large mammals of the mid Planas River basin, Colombia. Biota Colombiana 20(2):76-92. Baechli J, Albanesi S, & Bellis LM. 2021. Effectiveness of crossings as wildlife passages for mammals in the Yungas of Argentina. Journal of Nature Conservation 59:125944. https://doi.org/10.1016/j.jnc.2020.125944 . Bardeleben C, Moore RL, & Wayne RK. 2005. Isolation and Molecular Evolution of the Selenocysteine tRNA (Cf TRSP) and RNase P RNA (Cf RPPH1) Genes in the Dog Family, Canidae. Molecular Biology and Evolution 22(2):347-359. Beisiegel B de M. 1999. Contribuição ao estudo da história natural do cachorro do mato, Cerdocyon thous, e do cachorro vinagre, Speothos venaticus. PhD Dissertation. Instituto de Psicologia, Universidade de São Paulo, Brazil. Berta A. 1982. Cerdocyon thous. Mammalian Species 186(23):1-4. -

First Camera Trap Record of Bush Dogs in the State of São Paulo, Brazil
Beisiegel Bush dogs in S ão Paulo Canid News Copyright © 2009 by the IUCN/SSC Canid Specialist Group. ISSN 1478 -2677 The following is the established format for referencing this article: Beisiegel, B.M. 2009. First camera trap records of bush dogs in the state of S ão Paulo, Brazil. Canid News 12.5 [online] URL: http://www.canids.org/canidnews/12/Bush_dogs_in_Sao_Paulo.pdf. Field Report First camera trap record of bush dogs in the state of São Paulo, Brazil Beatriz M. Beisiegel Centro Nacional de Pesquisas para a Conservação dos PrePre dadores Naturais – CENAP / ICMBio. Av. dos Bandeirantes, s/n, Balneário Municipal, AtiAtibaibaia,a, CEP 12.941 -980, SP, Brazil. Email: [email protected] Keywords: Atlantic forest; camera trap ; sampling effort; Speothos venaticus . Abstract Thirteen carnivore species occur at the PECB, and a previous study using th ree camera traps (TM 500, Trail Master), over a two year period A picture of a bush dog Speothos venaticus pair obtained pictures of five of them ( crab-eating was obtained with a minimum sampling effort raccoon Procyon cancrivorus , puma Puma con- of 4,818 camera days, using seven to ten cam- color , ring-tailed coati Nasua nasua , neotropical era traps during 922 days at Parque Estadual river otter Lontra longicaudis and ocelot Leopar- Carlos Botelho, an Atlantic forest site. This dus pardalis - Beisiegel 1999). In the current picture confirms the presence of the species in study, a continuous sampling effort using the state of São Paulo, Brazil. seven to ten camera traps (Tigrinus 4.0 C, Br a- zil) begun in May 2006. -

Speothos Venaticus Across the Amazon and Other Biomes
How rare is rare? Quantifying and assessing the rarity of the bush dog Speothos venaticus across the Amazon and other biomes T ADEU G. DE O LIVEIRA,FERNANDA M ICHALSKI,ANDRÉ L. M. BOTELHO L INCOLN J. MICHALSKI,ARMANDO M. CALOURO and A RNAUD L. J. DESBIEZ Abstract The bush dog Speothos venaticus is a medium- Keywords Amazon, bush dog, camera trap, conservation, sized Neotropical canid. It is considered to be rare and its group size, rarity, relative abundance, Speothos venaticus biology and population parameters are still poorly under- To view supplementary material for this article, please visit stood. The Amazon is one of the main strongholds of this https://doi.org/./S species and is important for maintaining viable populations, as the region still holds extensive tracts of pristine habitat. We gathered field data from camera-trap studies throughout the Brazilian Amazon to estimate the relative abundance Introduction of the species and gain an understanding of its rarity, and how this compares with estimates from other vegetative for- he bush dog Speothos venaticus is a medium-sized mations and for sympatric hypercarnivores. We focused on T(c. kg) Neotropical canid that lives in packs. The spe- three pristine or partially disturbed sites and one fragmen- cies’ biology and population characteristics are poorly ted site. The estimated relative abundance of the species was understood, and it is one of the least known carnivores in – . individuals per trap-days, confirming that South America (Eisenberg & Redford, ; Zuercher ’ the species is rare. The bush dog s abundance in the et al., ; DeMatteo & Loiselle, ). -

Reconstructing Three Decades of Land Use and Land Cover Changes in Brazilian Biomes with Landsat Archive and Earth Engine
remote sensing Article Reconstructing Three Decades of Land Use and Land Cover Changes in Brazilian Biomes with Landsat Archive and Earth Engine Carlos M. Souza Jr. 1,* , Julia Z. Shimbo 2, Marcos R. Rosa 3 , Leandro L. Parente 4 , Ane A. Alencar 2 , Bernardo F. T. Rudorff 5 , Heinrich Hasenack 6 , Marcelo Matsumoto 7 , Laerte G. Ferreira 4, Pedro W. M. Souza-Filho 8 , Sergio W. de Oliveira 9, Washington F. Rocha 10 , Antônio V. Fonseca 1 , Camila B. Marques 2, Cesar G. Diniz 11 , Diego Costa 10 , Dyeden Monteiro 12, Eduardo R. Rosa 13 , Eduardo Vélez-Martin 6 , Eliseu J. Weber 14 , Felipe E. B. Lenti 2 , Fernando F. Paternost 13, Frans G. C. Pareyn 15, João V. Siqueira 16, José L. Viera 15, Luiz C. Ferreira Neto 11, Marciano M. Saraiva 5 , Marcio H. Sales 17, Moises P. G. Salgado 5 , Rodrigo Vasconcelos 10, Soltan Galano 10, Vinicius V. Mesquita 4 and Tasso Azevedo 18 1 Instituto do Homem e Meio Ambiente da Amazônia (Imazon), Belém 66055-200, Brazil; [email protected] 2 Instituto de Pesquisa Ambiental da Amazônia (Ipam), Brasília 70863-520, Brazil; [email protected] (J.Z.S.); [email protected] (A.A.A.); [email protected] (C.B.M.); [email protected] (F.E.B.L.) 3 Programa de Pós-Gradução em Geografia Física, Faculdade de Filosofia, Letras e Ciências Humanas, Universidade de São Paulo, São Paulo 05508-000, Brazil; [email protected] 4 Image Processing and GIS Laboratory (LAPIG), Federal University of Goiás (UFG), Goiania 74001-970, Brazil; [email protected] (L.L.P.); [email protected] (L.G.F.); [email protected]