Proceedings 2007 International Workshop on the Rehabilitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
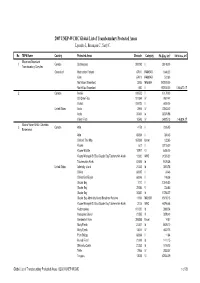
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C. No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Ellesmere/Greenland 1 Canada Quttinirpaaq 300093 II 38148.00 Transboundary Complex Greenland Hochstetter Forland 67910 RAMSAR 1848.20 Kilen 67911 RAMSAR 512.80 North-East Greenland 2065 MAB-BR 972000.00 North-East Greenland 650 II 972000.00 1,008,470.17 2 Canada Ivvavik 100672 II 10170.00 Old Crow Flats 101594 IV 7697.47 Vuntut 100673 II 4400.00 United States Arctic 2904 IV 72843.42 Arctic 35361 Ia 32374.98 Yukon Flats 10543 IV 34925.13 146,824.27 Alaska-Yukon-British Columbia 3 Canada Atlin 4178 II 2326.95 Borderlands Atlin 65094 II 384.45 Chilkoot Trail Nhp 167269 Unset 122.65 Kluane 612 II 22015.00 Kluane Wildlife 18707 VI 6450.00 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 12200 WHC 31595.00 Tatshenshini-Alsek 67406 Ib 9470.26 United States Admiralty Island 21243 Ib 3803.76 Chilkat 68395 II 24.46 Chilkat Bald Eagle 68396 II 198.38 Glacier Bay 1010 II 13045.50 Glacier Bay 22485 V 233.85 Glacier Bay 35382 Ib 10784.27 Glacier Bay-Admiralty Island Biosphere Reserve 11591 MAB-BR 15150.15 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 2018 WHC 66796.48 Kootznoowoo 101220 Ib 3868.24 Malaspina Glacier 21555 III 3878.40 Mendenhall River 306286 Unset 14.57 Misty Fiords 21247 Ib 8675.10 Misty Fjords 13041 IV 4622.75 Point Bridge 68394 II 11.64 Russell Fiord 21249 Ib 1411.15 Stikine-LeConte 21252 Ib 1816.75 Tetlin 2956 IV 2833.07 Tongass 13038 VI 67404.09 Global List of Transboundary Protected Areas ©2007 UNEP-WCMC 1 of 78 No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Tracy Arm-Fords Terror 21254 Ib 2643.43 Wrangell-St Elias 1005 II 33820.14 Wrangell-St Elias 35387 Ib 36740.24 Wrangell-St. -

Recent Noteworthy Findings of Fungus Gnats from Finland and Northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae)
Biodiversity Data Journal 2: e1068 doi: 10.3897/BDJ.2.e1068 Taxonomic paper Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae) Jevgeni Jakovlev†, Jukka Salmela ‡,§, Alexei Polevoi|, Jouni Penttinen ¶, Noora-Annukka Vartija# † Finnish Environment Insitutute, Helsinki, Finland ‡ Metsähallitus (Natural Heritage Services), Rovaniemi, Finland § Zoological Museum, University of Turku, Turku, Finland | Forest Research Institute KarRC RAS, Petrozavodsk, Russia ¶ Metsähallitus (Natural Heritage Services), Jyväskylä, Finland # Toivakka, Myllyntie, Finland Corresponding author: Jukka Salmela ([email protected]) Academic editor: Vladimir Blagoderov Received: 10 Feb 2014 | Accepted: 01 Apr 2014 | Published: 02 Apr 2014 Citation: Jakovlev J, Salmela J, Polevoi A, Penttinen J, Vartija N (2014) Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae). Biodiversity Data Journal 2: e1068. doi: 10.3897/BDJ.2.e1068 Abstract New faunistic data on fungus gnats (Diptera: Sciaroidea excluding Sciaridae) from Finland and NW Russia (Karelia and Murmansk Region) are presented. A total of 64 and 34 species are reported for the first time form Finland and Russian Karelia, respectively. Nine of the species are also new for the European fauna: Mycomya shewelli Väisänen, 1984,M. thula Väisänen, 1984, Acnemia trifida Zaitzev, 1982, Coelosia gracilis Johannsen, 1912, Orfelia krivosheinae Zaitzev, 1994, Mycetophila biformis Maximova, 2002, M. monstera Maximova, 2002, M. uschaica Subbotina & Maximova, 2011 and Trichonta palustris Maximova, 2002. Keywords Sciaroidea, Fennoscandia, faunistics © Jakovlev J et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

SECTION ONE: Background: Supply & Sources of Bear Products
SECTION ONE: Background: Supply & Sources of Bear Products Historical Perspective to the Bear Trade 16 Bear Farming 28 Profiles of Chinese bear farms 47 Current Restrictions on International Trade: CITES (Convention on International Trade in Endangered Species) 59 World Society for the Protection of Animals The Bear Bile Business 15 Historical Perspective to the Bear Trade Victor Watkins Traditonal Chinese Medicine and the growth of the modern trade in bear products The use of herbs to cure illness can be traced back over 4,000 years in China. The earliest medicinal literature (Shen-nong Ben Cao) dates back to 482 BC and records 365 types of medicinal issues. One of the most famous Chinese herbals, (Ben Cao Gang Mu) was written by Li Shi-zhen during the Ming dynasty (1590). This work lists 1,892 types of herbs used as medicine. In the above mentioned literature, animal ingredients make up less than 10% of the medicinal ingredients, and the majority of those animal parts are insects. There is very little use of mammal body parts listed in these early Chinese traditional medicines1. The use of bear parts in medicines in China dates back over 3,000 years. Medicinal uses for bear gall bladder first appeared in writing in the seventh century A.D. in the Materia Medica of Medicinal Properties2. The use of bear bile has since spread to other Asian countries such as Korea and Japan where it has been adopted for use in local traditional medicines. Plant and animal products which are selected for use in Chinese medicine are classified according to their properties. -

Heart Rate During Hyperphagia Differs Between Two Bear Species
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Brage INN Physiology Heart rate during hyperphagia differs royalsocietypublishing.org/journal/rsbl between two bear species Boris Fuchs1,†, Koji Yamazaki2,†, Alina L. Evans1, Toshio Tsubota3, Shinsuke Koike4,5, Tomoko Naganuma5 and Jon M. Arnemo1,6 Research 1Department of Forestry and Wildlife Management, Faculty of Applied Ecology and Agricultural Sciences, Cite this article: Fuchs B, Yamazaki K, Evans Inland Norway University of Applied Sciences, Campus Evenstad, 2418 Elverum, Norway 2Department of Forest Science, Tokyo University of Agriculture, 1-1-1 Sakuragaoka, Setagaya-Ku, Tokyo, Japan AL, Tsubota T, Koike S, Naganuma T, Arnemo 3Department of Environmental Veterinary Sciences, Faculty of Veterinary Medicine, Hokkaido University, Kita18, JM. 2019 Heart rate during hyperphagia differs Nishi9, Kita-Ku, Sapporo, Hokkaido, Japan between two bear species. Biol. Lett. 15: 4Institute of Global Innovation Research, and 5United Graduate School of Agricultural Science, Tokyo University 20180681. of Agriculture and Technology, 3-5-8 Saiwai, Fuchu-city, Tokyo, Japan 6Department of Wildlife, Fish and Environmental Studies, Faculty of Forest Sciences, Swedish University of http://dx.doi.org/10.1098/rsbl.2018.0681 Agricultural Sciences, 901 83, Umea˚, Sweden BF, 0000-0003-3412-3490; ALE, 0000-0003-0513-4887 Received: 28 September 2018 Hyperphagia is a critical part of the yearly cycle of bears when they gain fat reserves before entering hibernation. We used heart rate as a proxy to com- Accepted: 17 December 2018 pare the metabolic rate between the Asian black bear (Ursus thibetanus)in Japan and the Eurasian brown bear (Ursus arctos) in Sweden from summer into hibernation. -

Pasvik–Inari Nature and History Shared Area Description
PASVIK–INARI NATURE AND HISTORY SHARED AREA DESCRIPTION The Pasvik River flows from the largest lake in Finn- is recommended only for very experienced hikers, ish Lapland, Lake Inari, and extends to the Barents some paths are marked for shorter visits. Lake Inari Sea on the border of Norway and Russia. The valley and its tributaries are ideal for boating or paddling, forms a diverse habitat for a wide variety of plants and in winter the area can be explored on skis or a and animals. The Pasvik River is especially known for dog sled. The border mark at Muotkavaara, where its rich bird life. the borders of Finland, Norway and Russia meet, can The rugged wilderness that surrounds the river be reached by foot or on skis. valley astonishes with its serene beauty. A vast Several protected areas in the three neighbouring pine forest area dotted with small bogs, ponds and countries have been established to preserve these streams stretches from Vätsäri in Finland to Pasvik in great wilderness areas. A vast trilateral co-operation Norway and Russia. area stretching across three national borders, con- The captivating wilderness offers an excellent sisting of the Vätsäri Wilderness Area in Finland, the setting for hiking and recreation. From mid-May Øvre Pasvik National Park, Øvre Pasvik Landscape until the end of July the midnight sun lights up the Protection Area and Pasvik Nature Reserve in Nor- forest. The numerous streams and lakes provide way, and Pasvik Zapovednik in Russia, is protected. ample catch for anglers who wish to enjoy the calm backwoods. -

RCN #33 21/8/03 13:57 Page 1
RCN #33 21/8/03 13:57 Page 1 No. 33 Summer 2003 Special issue: The Transformation of Protected Areas in Russia A Ten-Year Review PROMOTING BIODIVERSITY CONSERVATION IN RUSSIA AND THROUGHOUT NORTHERN EURASIA RCN #33 21/8/03 13:57 Page 2 CONTENTS CONTENTS Voice from the Wild (Letter from the Editors)......................................1 Ten Years of Teaching and Learning in Bolshaya Kokshaga Zapovednik ...............................................................24 BY WAY OF AN INTRODUCTION The Formation of Regional Associations A Brief History of Modern Russian Nature Reserves..........................2 of Protected Areas........................................................................................................27 A Glossary of Russian Protected Areas...........................................................3 The Growth of Regional Nature Protection: A Case Study from the Orlovskaya Oblast ..............................................29 THE PAST TEN YEARS: Making Friends beyond Boundaries.............................................................30 TRENDS AND CASE STUDIES A Spotlight on Kerzhensky Zapovednik...................................................32 Geographic Development ........................................................................................5 Ecotourism in Protected Areas: Problems and Possibilities......34 Legal Developments in Nature Protection.................................................7 A LOOK TO THE FUTURE Financing Zapovedniks ...........................................................................................10 -

The Federal Nature Preserves (Zapovedniks) of Russia
MONITORING IN THE URAL RESERVES (ZAPOVEDNIKS) Kvashnina A.E. Zapovdnik “Denezhkin Kamen”, Sverdlovskaya Oblast, Severouralsk, Vsevolodo- Blagodatskoe, Russia, 624477 Marin Y.F., Mishin A.S. Visimskiy zapovednik, Sverdlovskaya Oblast, Kirovgrad, Stepan Razin St. 23, Russia, 624150 Loskutova N.M. Zapovednik “Basegi”, Permskaya Oblast, Gremiachinsk, Lenin St. 100, Russia, 618280 INTRODUCTION. The Federal Nature Preserves (Zapovedniks) of Russia. Russia and the former Soviet Union have been the scene of an unusually comprehensive attempt at biodiversity conservation through the establishment of an extensive network of protected natural areas. These natural areas include several categories of territory which today account in aggregate for some one-and-a-half percent of the land area of Russia. Territory categories include: zapovedniks - the strictly protected scientific Nature Reserves (World Conservation Union or IUCN category I State Nature Reserves or Scientific Reserves); National Parks - (IUCN category II); Natural Parks – (IUCN category V); zakazniks – natural refuges and wildlife sanctuaries (IUCN categories IV, V); natural monuments – small scale areas protecting unique biological objects (IUCN category III); arboreta (dendrological parks) and botanical gardens (Colwell et al., 1997). The zapovednik, or Russian Federal Nature Preserve, is a specially protected natural territory or aquatory that excludes all forms of management, even general visiting (except for the needs of research or protection), in order to preserve its indigenous complexes in their untouched natural state. At the same time, a zapovednik is an institution designed not just for the conservation of its territory but also for study. The principal tasks of the zapovedniks were formulated in the beginning of the last century by the Russian scientist Kozhevnikov (1909, 1911 and 1928) and by Dokuchaev (Shtilmark, 1996). -

From Sacred Cow to Cash Cow Muller, Martin
From sacred cow to cash cow Muller, Martin License: Creative Commons: Attribution-NoDerivs (CC BY-ND) Document Version Publisher's PDF, also known as Version of record Citation for published version (Harvard): Müller, M 2014, 'From sacred cow to cash cow: the shifting political ecologies of protected areas in Russia', Zeitschrift für Wirtschaftsgeographie, vol. 58, no. 2-3, pp. 127-143. Link to publication on Research at Birmingham portal General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. •User may use extracts from the document in line with the concept of ‘fair dealing’ under the Copyright, Designs and Patents Act 1988 (?) •Users may not further distribute the material nor use it for the purposes of commercial gain. Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document. When citing, please reference the published version. Take down policy While the University of Birmingham exercises care and attention in making items available there are rare occasions when an item has been uploaded in error or has been deemed to be commercially or otherwise sensitive. -

Action Plan Pasvik-Inari Trilateral Park 2019-2028
Action plan Pasvik-Inari Trilateral Park 2019-2028 2019 Action plan Pasvik-Inari Trilateral Park 2019-2028 Date: 31.1.2019 Authors: Kalske, T., Tervo, R., Kollstrøm, R., Polikarpova, N. and Trusova, M. Cover photo: Young generation of birders and environmentalists looking into the future (Pasvik Zapovednik, О. Кrotova) The Trilateral Advisory Board: FIN Metsähallitus, Parks & Wildlife Finland Centre for Economic Development, Transport and the Environments in Lapland (Lapland ELY-centre) Inari Municipality NOR Office of the Finnmark County Governor Øvre Pasvik National Park Board Sør-Varanger Municipality RUS Pasvik Zapovednik Pechenga District Municipality Nikel Local Municipality Ministry of Natural Resource and Ecology of the Murmansk region Ministry of Economic Development of the Murmansk region, Tourism division Observers: WWF Barents Office Russia, NIBIO Svanhovd Norway Contacts: FINLAND NORWAY Metsähallitus, Parks & Wildlife Finland Troms and Finnmark County Governor Ivalo Customer Service Tel. +47 789 50 300 Tel. +358 205 64 7701 [email protected] [email protected] Northern Lapland Nature Centre Siida RUSSIA Tel. +358 205 64 7740 Pasvik State Nature Reserve [email protected] (Pasvik Zapovednik) Tel./fax: +7 815 54 5 07 00 [email protected] (Nikel) [email protected] (Rajakoski) 2 Action Plan Pasvik-Inari Trilateral Park 2019-2028 3 Preface In this 10-year Action Plan for the Pasvik-Inari Trilateral Park, we present the background of the long-lasting nature protection and management cooperation, our mutual vision and mission, as well as the concrete development ideas of the cooperation for the next decade. The plan is considered as an advisory plan focusing on common long-term guidance and cooperation. -

Hubei Shennongjia
ASIA / PACIFIC HUBEI SHENNONGJIA CHINA Laojunshan Component of the property - © IUCN Bruce Jefferies China - Hubei Shennongjia WORLD HERITAGE NOMINATION – IUCN TECHNICAL EVALUATION HUBEI SHENNONGJIA (CHINA) – ID 1509 IUCN RECOMMENDATION TO WORLD HERITAGE COMMITTEE: To inscribe the property under natural criteria. Key paragraphs of Operational Guidelines: Paragraph 77: Nominated property meets World Heritage criteria. Paragraph 78: Nominated property meets integrity and protection and management requirements. 1. DOCUMENTATION S. and Hong Qian. Global Significance of Plant Diversity in China. In The Plants of China: A a) Date nomination received by IUCN: 16 March Companion to the Flora of China (2015). Huang, J. H., 2015 Chen, J.H., Ying, J.S., and Ke‐Ping M. Features and distribution patterns of Chinese endemic seed plant b) Additional information officially requested from species. Journal of Systematics and Evolution 49, no. and provided by the State Party: On 6 September 2 (2011): 81-94. Li, Y. (2004). The effect of forest 2015, the State Party responded to issues which arose clear-cutting on habitat use in Sichuan snub-nosed during the course of the IUCN field evaluation mission. monkey (Rhinopithecus roxellana) in Shennongjia The letter, with accompanying maps, addressed a Nature Reserve, China. Primates 45.1 69-72.. López- range of issues and confirmed extensions to the Pujol, J., et al. (2011). Mountains of Southern China as nominated area and buffer zone in the Badong County “plant museums” and “plant cradles”: evolutionary and area. Following the IUCN World Heritage Panel a conservation insights. Mountain Research and progress report was sent to the State Party on 16 Development,31(3), 261-269. -

THE PUTORANSKY STATE NATURE RESERVE (Taimyr Peninsula)
Business overview Corporate resposibility Environment THE PUTORANSKY STATE NATURE RESERVE (Taimyr Peninsula) Norilsk Area 1,887 thousand ha In 2017, the Putoransky State Nature Reserve kept implementing projects selected under Nornickel’s World of New Opportunities charitable programme. Save the Bighorn Together Norilsk Lakes to Norilsk People An ambitious programme to protect the endangered Norilsk Lakes to Norilsk People project implemented species of the bighorn found in the Putoranа Plateau since 2013 seeks to preserve the Big Norilsk only and listed on Russia’s Red Data Book. The Lakes, a unique ecosystem of subarctic mountains. Company provides funding for volunteer training at During that time, Nornickel provided funding for the the Surveillance School, ground research to collect recreational fisheries in the upper part of the Pyasina data on the bighorn population, and Putorany. River basin, tourist and trekking infrastructure, Bighorn. People festival of friends. The project’s construction of a camping station at Lama Lake and funding totals some USD 86,000 (RUB 4.99 mln). a base station at Sobachye Lake. In 2017, as part of the project, the Company allocated over USD 17,000 (around RUB 1 mln) to finance an environmental and educational summer camp at Lake Lama for students of the volunteer school. • 142 • • 143 • Annual report • 2017 THE PASVIK NATURE RESERVE Company overview (Kola Peninsula) Nickel Strategy overview Strategy Area 14.6 thousand ha Market overview Market Business overview Business overview The Pasvik Nature Reserve is home to rare species Nornickel supports scientific research carried out by listed on the international and Russia’s Red Data the nature reserve, its efforts to protect natural and Books. -

Protected Areas and Beyond
MAGAZINE Protection in a time of change 7 Economic values 16 No. 3 2012 ThE CirclE lessons from elsewhere 27 PROTECTED AREAS AND BEYOND PUBLISHED BY THE WWF GLoBaL aRCTIC PRoGRaMME ThE CiRClE 3.2012 Contents EDITORIAL What is beyond protected areas? 3 In BRIEf 4 TOM BARRY, COURTnEY PRICE Arctic protected areas: conservation in a time of change 6 LISA SPEER, TOM LAUGHLIn EBSAs in the arctic marine environment 12 n IK LOPOUKHInE Protected areas and management of the Arctic 14 n IGEL DUDLEY Economic values of protected areas and other natural landscapes in the Arctic 16 BE nTE CHRISTIAnSEn, TIIA KALSKE Environmental protection in the Pasvik-Inari area 18 An n A KUHMOnEn, OLEG SUTKAITIS Representative network of protected areas to conserve northern nature 20 KARE n MURPHY, LISA MATLOCK Improving Alaska’s wildlife conservation through applied science 22 ZOLTA n KUn Supporting local development beyond commodities 24 ALBERTO ARROYO SCHnELL The natura 2000 network: where ecology and economy meet 27 CRISTIA n MOnTALVO MAnCHEnO Development of an ecological network approach in the Caucasus 28 ALEXA nDER SHESTAKOV A Pan-Arctic Ecological network: the concept and reality 30 TE H PICTURE 32 PROTECTED AREAS AND BEYOND Photo: Kitty Terwolbeck, Flickr/Creative commons Terwolbeck, Photo: Kitty The Circle is published quar- Publisher: Editor in Chief: Clive Tesar, COVER: Cotton grass in Sarek terly by the WWF Global Arctic WWF Global Arctic Programme [email protected] National Park in Sweden, one of Programme. Reproduction and 30 Metcalfe Street Editor: Lena Eskeland, [email protected] Europe's oldest parks. quotation with appropriate credit Suite 400 Photo: Kitty Terwolbeck, Flickr/Creative commons are encouraged.