The Platypus: Historical, Ecological, and Behavioural Advances to Improve the Conservation of an Elusive Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

November 18–20, 2016 Lake Crackenback Resort & Spa Trextriathlon.Com.Au Welcome from the NSW Government
#GetDirtyDownUnder #TreXTri presented by November 18–20, 2016 Lake Crackenback Resort & Spa trextriathlon.com.au Welcome from the NSW Government On behalf of the NSW Government I’d like to invite you to Lake Crackenback Resort & Spa in New South Wales, Australia, for the 2016 ITU World Cross Triathlon Championships, to be held in November next year. The NSW Government is proud to have secured the World Cross Triathlon Championships for the Snowy Mountains, through our tourism and major events agency Destination NSW in partnership with In2Adventure and Triathlon Australia. The Snowy Mountains is an ideal host for the World Championships, and I am sure that visiting competitors will be enthralled by the region’s breathtaking beauty. The Snowy Mountains has everything you would want from an adventure sports location, from stunning mountain bike trails to pristine lakes, with plenty of space to compete, train or just explore. I encourage all visitors to the Snowy Mountains to take some time to experience everything the region has to offer, with top class restaurants, hotels and attractions as well as the inspiring landscapes. New South Wales also has much more to offer competitors and visitors, from our global city, Sydney, to our spectacular coastline and wide variety of natural landscapes. I wish all competitors the best of luck in Sardinia and we look forward to welcoming you all to New South Wales for the 2016 ITU World Cross Triathlon Championships. Stuart Ayres Minister for Trade, Tourism and Major Events Minister for Sport 1 Sydney is a city on the move, with exciting new harbourside precincts featuring world-class hotels and sleek shopping districts. -
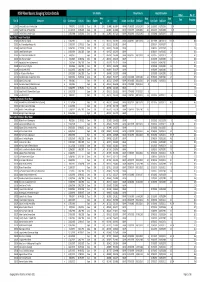
Gauging Station Index
Site Details Flow/Volume Height/Elevation NSW River Basins: Gauging Station Details Other No. of Area Data Data Site ID Sitename Cat Commence Ceased Status Owner Lat Long Datum Start Date End Date Start Date End Date Data Gaugings (km2) (Years) (Years) 1102001 Homestead Creek at Fowlers Gap C 7/08/1972 31/05/2003 Closed DWR 19.9 -31.0848 141.6974 GDA94 07/08/1972 16/12/1995 23.4 01/01/1972 01/01/1996 24 Rn 1102002 Frieslich Creek at Frieslich Dam C 21/10/1976 31/05/2003 Closed DWR 8 -31.0660 141.6690 GDA94 19/03/1977 31/05/2003 26.2 01/01/1977 01/01/2004 27 Rn 1102003 Fowlers Creek at Fowlers Gap C 13/05/1980 31/05/2003 Closed DWR 384 -31.0856 141.7131 GDA94 28/02/1992 07/12/1992 0.8 01/05/1980 01/01/1993 12.7 Basin 201: Tweed River Basin 201001 Oxley River at Eungella A 21/05/1947 Open DWR 213 -28.3537 153.2931 GDA94 03/03/1957 08/11/2010 53.7 30/12/1899 08/11/2010 110.9 Rn 388 201002 Rous River at Boat Harbour No.1 C 27/05/1947 31/07/1957 Closed DWR 124 -28.3151 153.3511 GDA94 01/05/1947 01/04/1957 9.9 48 201003 Tweed River at Braeside C 20/08/1951 31/12/1968 Closed DWR 298 -28.3960 153.3369 GDA94 01/08/1951 01/01/1969 17.4 126 201004 Tweed River at Kunghur C 14/05/1954 2/06/1982 Closed DWR 49 -28.4702 153.2547 GDA94 01/08/1954 01/07/1982 27.9 196 201005 Rous River at Boat Harbour No.3 A 3/04/1957 Open DWR 111 -28.3096 153.3360 GDA94 03/04/1957 08/11/2010 53.6 01/01/1957 01/01/2010 53 261 201006 Oxley River at Tyalgum C 5/05/1969 12/08/1982 Closed DWR 153 -28.3526 153.2245 GDA94 01/06/1969 01/09/1982 13.3 108 201007 Hopping Dick Creek -

Snowy River National Park Visitor Guide
Snowy River National Park Visitor Guide Reserved as a national park in 1979, Snowy River National Park now covers 114,505 ha. Named after the Snowy River, which bisects the park as it cuts through Tulloch Ard Gorge on its way from the alps to the sea, the park features spectacular river scenery, magnificent deep gorges, old growth forests dominated by towering Alpine Ash and rain‐shadow Cypress Pine woodlands and pristine wilderness areas. Welcome to Country Raymond Creek Falls – 1km, 30 minutes one way In 2010 the Federal Government recognised the Gunaikurnai’s native title rights over much of Gippsland. This included much of the These picturesque falls in the south of the park can be reached all Snowy River National Park, west of the Snowy River. At the same year round from Orbost along Moorsford Road. A short walking time the Victorian Government entered into legal agreements under track leads to a view of Raymond Creek Falls plunging 20 metres into the Traditional Owner Settlement Act in acknowledgement of a deep, clear pool. To get another perspective, continue further Gunaikurnai’s traditional owner rights, known as Joint Management 300m to the bottom of the falls. of ten parks and reserves in Gippsland. Through Joint Management, the State has created an equal Tulloch Ard Gorge Lookout – 1.6km, 1.5hours return partnership with the Gunaikurnai people. The benefits of this partnership are many and include: the knowledge and culture of This walk takes you through diverse vegetation culminating in the Traditional Owners incorporated into natural resources (land) observation lookout offering spectacular views of the Snowy River management, cultural awareness and knowledge of local history, country and Tulloch Ard Gorge. -

Snowy River Basin January 2014
Snowy River Basin January 2014 Introduction Southern Rural Water is the water corporation responsible for administering and enforcing the Snowy River Basin Local Management Plan. The purpose of the Snowy River Basin Local Management Plan is to: • document the management objectives for the system • explain to licence holders (and the broader community) the specific management objectives and arrangements for their water resource and the rules that apply to them as users of that resource; • clarify water sharing arrangements for all users and the environment, including environmental flow requirements • document any limits, including water use caps, permissible consumptive volumes or extraction limits that apply to the system. Management objectives The objective of the Local Management Plan is to ensure the equitable sharing of water between users and the environment and the long-term sustainability of the resource. Water system covered The Local Management Plan covers all the rivers and creeks located within the Snowy River Basin, which includes: • Snowy River • Major Creek • Brodribb River • Buchan River • Deddick River • Cabbage Tree Creek • Hospital Creek The Snowy River Basin is shown in the map below. Catchment information The Snowy River flows across the NSW border to the coast at Marlo. Significant tributaries of the Snowy River include Buchan, Murrindal, Suggan Buggan, Deddick and Brodribb Rivers. The Snowy floodplain contains a complex system of drains and levee banks to support agricultural production. Heritage River status applies to the entire Victorian length of the Snowy River. The Snowy has many significant attributes including canoeing and rafting opportunities, scenic landscapes, cultural Indigenous heritage sites and native fish and fauna habitat. -

The Canberra • B Ush Walking Club ( Inc. Newsletter
THE CANBERRA • B USH WALKING CLUB ( INC. NEWSLETTER GPO Box 160, Canberra ACT 2601 VOLUME 36 October 2000 NUMBER 10 OCTOBER GENERAL MEETING 8pm Wednesday 18th Speaker: Betty Kitchener, on 'Field First Aid' Woden Library Community Room Make the most of the evening and join other members at 6. OOpm for a convivial meal at the Chinese Kitchen 6)10 Restaurant in Corinna Street, Shop 091, Woden Plaza, Phi/lip. to be early to ensure there will be ample time to finish and still get to the meeting in good ti PRESIDENT'S • Membership fees have been increased to $25 (single) and Also In This Issue: PRATTLE $33 (household) Item Page • The Club transport rate has PRESIDENT'S PRATTLE For those of you who were unable been increased to to make last month's Annual Gen- MEMBERSHIP MATTERS 2 30cents/kilometrelvehicle. eral Meeting, the key outcomes are MOTIONS PASSED AT AGM 2 as follows: Contact details for the Committee " are shown on the back page of each 39 ANNUAL REPORT 2 We have four brand new Com- It. Please don't hesitate to give us a CBC 40th ANNIVERSARY 4 mittee members - Ailsa Brown call if you have concerns about the TRIP PREVIEWS 4 (Publisher), Michael Macona- way we are doing things or have chie (Conservation Officer), some suggestions for how we might WALKS WAFFLE 5 Michael Sutton (Treasurer), do things better. A bit of praise LETTERS TO THE EDITOR. 6 and Rosanne Walker (Social from time to time helps keep us TRIP REPORTS 7 Secretary), replacing Vance going so do let us know if we do Brown, Janet Edstein, Cate something that pleases you. -

Cooma-Monaro Shire Development Control Plan 2014 (Amendment 2)
Cooma-Monaro Shire Development Control Plan 2014 (Amendment 2) 1 | P a g e Draft Development Control Plan Amendments Cooma-Monaro Development Control Plan MONARO SHIRE COUNCIL D evelopment Control Plan 2014 As adopted by Council on 17 October 2019 (Resolution 362/19) Came into force on 11/11/2019 Plan 2014 COOMA - Record of Versions Name of Amendment Adopted by Council on Resolution Came into Force on (date of notice in local press) Original (Issue 1) 14/04/2014 129/14 17/04/2014 Amendment 1 (Issue 3) 14/03/2016 58/16 23/03/2016 Amendment 2 (Issue 4) 17/10/2019 362/19 11/11/2019 H:\flightplan\ServicePages\Documentation\Policies\Urban & Rural Planning\DCP\_WordDoc\Cooma-Monaro Shire Development Control Plan 2014.docx 2 | P a g e Draft Development Control Plan Amendments Cooma-Monaro Development Control Plan MONARO SHIRE COUNCIL D evelopment Control Plan 2014 Contents 1 Introduction 9 1.1 How to use this Plan 10 1.2 Structure of this Plan 10 Plan 2014 1.3 Relationship to other plans 11 1.4 Name of this Plan 12 1.5 Land to which this Plan applies 12 1.6 Commencement date 12 1.7 Aims of this Plan 12 1.8 Categories of development 12 1.9 Variations to development standards 13 1.10 Is development consent required? 14 2 General Development Controls 16 2.1 Streetscape 16 2.1.1 Objectives 16 COOMA 2.1.2 Performance based requirements 17 2.1.3 Prescriptive requirements 17 2.2 Building height and bulk 17 - 2.2.1 Objectives 18 2.2.2 Performance based requirements 18 2.2.3 Prescriptive requirements 18 2.3 Building setbacks 19 2.3.1 Objectives 19 2.3.2 -

Kosciuszko National Park Thredbo–Perisher Area Bike Trails
Photo: Thredbo Valley track (Thredbo Resort) Kosciuszko National Park Thredbo–Perisher area bike trails The Thredbo–Perisher area is one of mountain bike ride. The trail follows the old road to Australia’s premier mountain biking Mount Kosciuszko, which closed to public vehicles destinations. From leisurely cycles, to in 1976 due to safety and environmental concerns. cross-country and adrenaline trails, Pass through snow gums, heath and herb fields there’s something for everyone. and enjoy expansive views of the Main Range. Cross the Snowy River and climb the winding Plan with weather and track conditions in trail to Seamans Hut, which was built in 1929 mind. Snow can fall at any time of year, as a memorial to skiers Laurie Seaman and covering the tracks and bringing freezing Evan Hayes. conditions. Some rides can only be enjoyed when there’s no snow – check with our visitor You’ll need to leave your bike at Rawson Pass and centres before setting out. walk the 1.7km track to the summit – so carry a bike lock. The road has some steep sections but Remember to give way to walkers on all trails. the return leg is mostly downhill. Go slowly and be aware of walkers. ALPINE AREA TRAILS THREDBO AREA TRAILS When the winter snow melts, you’ll discover an ancient landscape of granite tors, glacial Ride beside cool mountain streams to lakes and summer wildflowers. historic huts, experience the thrill of a single track, downhill ride, or explore the Alpine Topographic maps Village of Thredbo. • Perisher Valley 1:25 000 • Youngal 1:25 000 Topographic -

Government Gazette of the STATE of NEW SOUTH WALES Number 52 Friday, 13 April 2007 Published Under Authority by Government Advertising
2217 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 52 Friday, 13 April 2007 Published under authority by Government Advertising SPECIAL SUPPLEMENT New South Wales Shoalhaven Local Environmental Plan 1985 (Amendment No 212)—Heritage under the Environmental Planning and Assessment Act 1979 I, the Minister for Planning, make the following local environmental plan under the Environmental Planning and Assessment Act 1979. (W97/00064/PC) FRANK SARTOR, M.P., MinisterMinister forfor PlanningPlanning e03-407-09.p04 Page 1 2218 SPECIAL SUPPLEMENT 13 April 2007 Shoalhaven Local Environmental Plan 1985 (Amendment No 212)— Clause 1 Heritage Shoalhaven Local Environmental Plan 1985 (Amendment No 212)—Heritage under the Environmental Planning and Assessment Act 1979 1 Name of plan This plan is Shoalhaven Local Environmental Plan 1985 (Amendment No 212)—Heritage. 2Aims of plan This plan aims: (a) to identify and conserve the environmental heritage of the City of Shoalhaven, and (b) to conserve the heritage significance of existing significant fabric, relics, settings and views associated with the heritage significance of heritage items and heritage conservation areas, and (c) to ensure that archaeological sites and places of Aboriginal heritage significance are conserved, and (d) to ensure that the heritage conservation areas throughout the City of Shoalhaven retain their heritage significance. 3 Land to which plan applies This plan applies to all land within the City of Shoalhaven under Shoalhaven Local Environmental Plan 1985. 4 Amendment of Shoalhaven Local Environmental Plan 1985 Shoalhaven Local Environmental Plan 1985 is amended as set out in Schedule 1. Page 2 NEW SOUTH WALES GOVERNMENT GAZETTE No. -

Mulloon Creek Baseline Fish Survey Autumn 2016
Mulloon Creek Baseline Fish Survey Autumn 2016 Final report to the Mulloon Institute Institute for Applied Ecology University of Canberra Acknowledgements The authors of this report wish to acknowledge the input, guidance and field assistance provided by Luke Peel. Fish were sampled under NSW Department of Primary Industries Scientific Collection Permit No: P07/0007-5.0. The Mulloon Institute wish to acknowledge the South East Local Land Services in funding of this baseline fish survey, and advice from NSW DPI Fisheries. Cite this report as follows: Starrs, D. and M. Lintermans (2016) Mulloon Creek baseline fish survey. Autumn 2016. Final report to the Mulloon Institute. Institute for Applied Ecology, University of Canberra, Canberra. 2 Table of Contents Acknowledgements ................................................................................................................................ 2 Table of Contents ................................................................................................................................... 3 Introduction ............................................................................................................................................ 4 Methods.................................................................................................................................................. 6 Results .................................................................................................................................................. 10 Discussion ........................................................................................................................................... -

Kilmore East Murrindindi Complex South Fire
KILMORE EAST MURRINDINDI COMPLEX SOUTH FIRE BURNED AREA EMERGENCY STABILIZATION PLAN BIODIVERSITY - FAUNA ASSESSMENT I. OBJECTIVES • Assess the effects of fire and suppression actions to the Threatened and Endangered Species of Victoria, Australia under the Flora and Fauna Guarantee Act 1988 (FFG Act), and the Environment Protection and Biodiversity Conservation Act 1999 • Prescribe emergency stabilization and rehabilitation measures and/or monitoring and assess the effects of these actions to listed species and their designated habitat. II. ISSUES Impacts to Rare or Threatened Species- Seven listed species under the Flora and Fauna Guarantee Act 1988 (FFG Act), (Leadbeater’s Possum [Gymnobelideus leadbeateri], Spotted tree-frog [Litora spenceri], Barred Galaxias [Galaxias olidus var. fuscus], Macquarie Perch (Macquaria australasica), Brush-tailed Phascogale [Phascogale tapoatafa], Powerful Owl [Ninox strenua]), Sooty Owl [Tyto tenebricosa], occur within the fire areas. Leadbeater’s Possum, Barred Galaxias, and Macquarie Perch, are also listed nationally under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) within the fire area. Impacts to these species and their habitats from the fire, suppression actions, and proposed emergency stabilization actions are addressed. III. OBSERVATIONS The purpose of this Burned Area Emergency Response (BAER) Wildlife Assessment is to document the effects of the fire, suppression activities, proposed stabilization treatments, and potential post fire flooding and sediment delivery to listed threatened and endangered fauna species and their preferred habitats within the fire area. This assessment includes effects to species that occur on lands under the tenure of the Department of Sustainability and Environment, Parks Victoria, Goulburn Broken Water Catchment Management Authority, Goulburn Valley Water, Melbourne Water Corporation, and private ownership. -

Rivers and Streams Special Investigation Final Recommendations
LAND CONSERVATION COUNCIL RIVERS AND STREAMS SPECIAL INVESTIGATION FINAL RECOMMENDATIONS June 1991 This text is a facsimile of the former Land Conservation Council’s Rivers and Streams Special Investigation Final Recommendations. It has been edited to incorporate Government decisions on the recommendations made by Order in Council dated 7 July 1992, and subsequent formal amendments. Added text is shown underlined; deleted text is shown struck through. Annotations [in brackets] explain the origins of the changes. MEMBERS OF THE LAND CONSERVATION COUNCIL D.H.F. Scott, B.A. (Chairman) R.W. Campbell, B.Vet.Sc., M.B.A.; Director - Natural Resource Systems, Department of Conservation and Environment (Deputy Chairman) D.M. Calder, M.Sc., Ph.D., M.I.Biol. W.A. Chamley, B.Sc., D.Phil.; Director - Fisheries Management, Department of Conservation and Environment S.M. Ferguson, M.B.E. M.D.A. Gregson, E.D., M.A.F., Aus.I.M.M.; General Manager - Minerals, Department of Manufacturing and Industry Development A.E.K. Hingston, B.Behav.Sc., M.Env.Stud., Cert.Hort. P. Jerome, B.A., Dip.T.R.P., M.A.; Director - Regional Planning, Department of Planning and Housing M.N. Kinsella, B.Ag.Sc., M.Sci., F.A.I.A.S.; Manager - Quarantine and Inspection Services, Department of Agriculture K.J. Langford, B.Eng.(Ag)., Ph.D , General Manager - Rural Water Commission R.D. Malcolmson, M.B.E., B.Sc., F.A.I.M., M.I.P.M.A., M.Inst.P., M.A.I.P. D.S. Saunders, B.Agr.Sc., M.A.I.A.S.; Director - National Parks and Public Land, Department of Conservation and Environment K.J. -

The Geology and Prospectivity of the Tallangatta 1:250 000 Sheet
VIMP Report 10 The geology and prospectivity of the Tallangatta 1:250 000 sheet I.D. Oppy, R.A. Cayley & J. Caluzzi November 1995 Bibliographic reference: OPPY, I.D., CAYLEY R.A. & CALUZZI, J., 1995. The Geology and prospectivity of the Tallangatta 1:250 000 sheet Victorian Initiative for Minerals and Petroleum Report 10. Department of Agriculture, Energy and Minerals. © Crown (State of Victoria) Copyright 1995 Geological Survey of Victoria ISSN 1323 4536 ISBN 0 7306 7980 2 This report may be purchased from: Business Centre, Department of Agriculture, Energy & Minerals, Ground Floor, 115 Victoria Parade, Fitzroy, Victoria 3065 For further technical information contact: General Manager, Geological Survey of Victoria, Department of Agriculture, Energy & Minerals, P O Box 2145, MDC Fitzroy, Victoria 3065 Acknowledgments: The authors wish to acknowledge G. Ellis for formatting the document, R. Buckley, P.J. O'Shea and D.H. Taylor for editing and S. Heeps for cartography I. Oppy wrote chapters 3 and 5, R. Cayley wrote chapter 2 and J. Caluzzi wrote chapter 4. GEOLOGY AND PROSPECTIVITY - TALLANGATTA 1 Contents Abstract 4 1 Introduction 5 2 Geology 7 2.1 Geological history 7 Pre-Ordovician to Early Silurian 7 Early Silurian Benambran deformation and widespread granite intrusion 8 Middle to Late Silurian 9 Late Silurian Bindian deformation 9 Early Devonian rifting and volcanism 10 Middle Devonian Tabberabberan deformation 11 Late Devonian sedimentation and volcanism 11 Early Carboniferous Kanimblan deformation to Present day 11 2.2 Stratigraphy