The Physiological Study of Emotional Piloerection: a Review and Guide for Future Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update
The ASAM NATIONAL The ASAM National Practice Guideline 2020 Focused Update Guideline 2020 Focused National Practice The ASAM PRACTICE GUIDELINE For the Treatment of Opioid Use Disorder 2020 Focused Update Adopted by the ASAM Board of Directors December 18, 2019. © Copyright 2020. American Society of Addiction Medicine, Inc. All rights reserved. Permission to make digital or hard copies of this work for personal or classroom use is granted without fee provided that copies are not made or distributed for commercial, advertising or promotional purposes, and that copies bear this notice and the full citation on the fi rst page. Republication, systematic reproduction, posting in electronic form on servers, redistribution to lists, or other uses of this material, require prior specifi c written permission or license from the Society. American Society of Addiction Medicine 11400 Rockville Pike, Suite 200 Rockville, MD 20852 Phone: (301) 656-3920 Fax (301) 656-3815 E-mail: [email protected] www.asam.org CLINICAL PRACTICE GUIDELINE The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update 2020 Focused Update Guideline Committee members Kyle Kampman, MD, Chair (alpha order): Daniel Langleben, MD Chinazo Cunningham, MD, MS, FASAM Ben Nordstrom, MD, PhD Mark J. Edlund, MD, PhD David Oslin, MD Marc Fishman, MD, DFASAM George Woody, MD Adam J. Gordon, MD, MPH, FACP, DFASAM Tricia Wright, MD, MS Hendre´e E. Jones, PhD Stephen Wyatt, DO Kyle M. Kampman, MD, FASAM, Chair 2015 ASAM Quality Improvement Council (alpha order): Daniel Langleben, MD John Femino, MD, FASAM Marjorie Meyer, MD Margaret Jarvis, MD, FASAM, Chair Sandra Springer, MD, FASAM Margaret Kotz, DO, FASAM George Woody, MD Sandrine Pirard, MD, MPH, PhD Tricia E. -

SUBLOCADE Education Brochure | SUBLOCADE® (Buprenorphine Extended-Release) Injection, for Subcutaneous Use (CIII) KEEP MOVING TOWARDS RECOVERY with Once-Monthly
SUBLOCADE® (buprenorphine extended-release) injection, for subcutaneous use (CIII) is a prescription medicine used to treat adults with moderate to severe addiction (dependence) to opioid drugs (prescription or illegal) who have received an oral transmucosal (used under the tongue or inside the cheek) buprenorphine-containing medicine at a dose that controls withdrawal symptoms for at least 7 days. SUBLOCADE is part of a complete treatment plan that should include counseling. SUBLOCADE Education Brochure | SUBLOCADE® (buprenorphine extended-release) injection, for subcutaneous use (CIII) KEEP MOVING TOWARDS RECOVERY with once-monthly Individuals depicted are models used for illustrative purposes only. IMPORTANT SAFETY INFORMATION Because of the serious risk of potential harm or death from self-injecting SUBLOCADE into a vein (intravenously), it is only available through a restricted program called the SUBLOCADE REMS Program. • SUBLOCADE is not available in retail pharmacies. • Your SUBLOCADE injection will only be given to you by a certified healthcare provider. Please see SUBLOCADE full Prescribing Information including BOXED WARNING and Medication Guide at sublocade.com, or included in the back of this brochure. Opioid addiction may be an overwhelming problem. But don’t give up. There are different ways to tackle it. Living with opioid addiction can be a struggle. But it’s important Opioid addiction is actually a disease called Opioid Use Disorder Medication-assisted treatment (MAT), which combines to understand that even when someone tries again and again (OUD), and it involves compulsive drug seeking and use, despite medication and counseling, is an option that can help manage to quit, it’s not a sign of weakness or failure. -

Appendix D: Important Facts About Alcohol and Drugs
APPENDICES APPENDIX D. IMPORTANT FACTS ABOUT ALCOHOL AND DRUGS Appendix D outlines important facts about the following substances: $ Alcohol $ Cocaine $ GHB (gamma-hydroxybutyric acid) $ Heroin $ Inhalants $ Ketamine $ LSD (lysergic acid diethylamide) $ Marijuana (Cannabis) $ MDMA (Ecstasy) $ Mescaline (Peyote) $ Methamphetamine $ Over-the-counter Cough/Cold Medicines (Dextromethorphan or DXM) $ PCP (Phencyclidine) $ Prescription Opioids $ Prescription Sedatives (Tranquilizers, Depressants) $ Prescription Stimulants $ Psilocybin $ Rohypnol® (Flunitrazepam) $ Salvia $ Steroids (Anabolic) $ Synthetic Cannabinoids (“K2”/”Spice”) $ Synthetic Cathinones (“Bath Salts”) PAGE | 53 Sources cited in this Appendix are: $ Drug Enforcement Administration’s Drug Facts Sheets1 $ Inhalant Addiction Treatment’s Dangers of Mixing Inhalants with Alcohol and Other Drugs2 $ National Institute on Alcohol Abuse and Alcoholism’s (NIAAA’s) Alcohol’s Effects on the Body3 $ National Institute on Drug Abuse’s (NIDA’s) Commonly Abused Drugs4 $ NIDA’s Treatment for Alcohol Problems: Finding and Getting Help5 $ National Institutes of Health (NIH) National Library of Medicine’s Alcohol Withdrawal6 $ Rohypnol® Abuse Treatment FAQs7 $ Substance Abuse and Mental Health Services Administration’s (SAMHSA’s) Keeping Youth Drug Free8 $ SAMHSA’s Center for Behavioral Health Statistics and Quality’s (CBHSQ’s) Results from the 2015 National Survey on Drug Use and Health: Detailed Tables9 The substances that are considered controlled substances under the Controlled Substances Act (CSA) are divided into five schedules. An updated and complete list of the schedules is published annually in Title 21 Code of Federal Regulations (C.F.R.) §§ 1308.11 through 1308.15.10 Substances are placed in their respective schedules based on whether they have a currently accepted medical use in treatment in the United States, their relative abuse potential, and likelihood of causing dependence when abused. -

Goose Bumps: Phenomenon of Spirituality and Body-Mind Cleansing?
September, 2011 Volume 11, No. 3 Goose bumps: Phenomenon of spirituality and body-mind cleansing? By Floco Tausin Abstract Many of us experience that prickly feeling with our body hair standing on end and our skin in goose bumps at various times. This is often associated with chills, shivers and certain emotional states. Less known, however, is that this prickle is informative and effective in the fields of health care and spirituality. This is suggested by both medical studies and the experience of spiritual masters from various cultures. Key words: goose bumps, hair standing on end, prickly sensations, holistic healing, spirituality The spiritual significance of prickling If the spiritual dimension of the prickly feeling is not immediately evident to us, it may be because our familiar sources of information don’t make ties between the two. According to current understandings of physiology, goose bumps are a relic of a distant past, when the hominids of prehistoric times were still covered with dense hair. The erection of the hair provided protection from the cold and made them look bigger and more menacing – which may have helped averting combats in threatening situations. For relatively hairless and clean-shaven modern people, however, these erections of sparse body hair and prickly feeling have lost these functions (Bubenick, 2003; cf. Gieler, 2002). Figure 1. Subjective aspects of prickly feelings, however, remain. The Bible, for Example from the instance, links this phenomenon to fear and terror: “Fear came upon popular media: me, and trembling, which made all my bones to shake. Then a spirit Ducks experiencing passed before my face; the hair of my flesh stood up.“ (Job 4,14-15) goose bumps to harmonious music (cf. -

Informationofpc Bulletin November 2009 Winter Weather Hazard Reminder
informationOFPC bulletin November 2009 Winter Weather Hazard Reminder Wind Chill, Frost Bite, and Hypothermia are three hazards to firefighters during winter/cold weather operations. Now is the time to prepare equipment, apparatus, and personnel for cold weather operations. Wind chill is the apparent temperature felt on exposed skin due to wind. The degree of this phenomenon depends on both air temperature and wind speed. The wind chill temperature (often popularly called the wind chill factor) is always lower than the air temperature for values where the wind chill formula is valid. The human body loses heat largely by evaporative cooling and convection. The rate of heat loss by a surface depends on the wind speed above that surface: the faster the wind speed, the more readily the surface cools. For inanimate objects, the effect of wind chill is to reduce any warmer objects to the ambient temperature more quickly. For most biological organisms, the physiological response is to maintain surface temperature in an acceptable range so as to avoid adverse effects. Thus, the attempt to maintain a given surface temperature in an environment of faster heat loss results in both the perception of lower temperatures and an actual greater heat loss increasing the risk to adverse effects such as frostbite and death. Frostbite (congelatio in medical terminology) is the medical condition wherein localized damage is caused to skin and other tissues due to extreme cold. Frostbite is most likely to happen in body parts farthest from the heart and those with large exposed areas. The initial stages of frostbite are sometimes called “frostnip”. -

Detection and Management of Acute Opioid Withdrawal in Non-Pregnant Patients Prescribed Opioids for Chronic Pain
Opioid Advice: Detection and Management of Acute Opioid Withdrawal in Non-Pregnant Patients Prescribed Opioids for Chronic Pain Context Withdrawal occurs in patients taking opioids regularly when: • the dose is reduced, missed or stopped • the patient is given a partial agonist or antagonist that precipitates withdrawal • opioids are switched or tapered • the patient voluntarily stops opioids. In patients with chronic pain, withdrawal may also occur between doses of opioid medications, often manifest as irritability and/or generalized pain prior to the next dose. This is particularly evident in the morning. Note: • Withdrawal does not necessarily indicate that the person is addicted. However, withdrawal does indicate physical dependence. • It is sometimes difficult to discern who is misusing prescription opioids. Health care providers need to pay attention to all patients. Signs/Symptoms Description of Opioid Withdrawal: Upregulated mu-opioid receptors that are not occupied by opioid agonists lead to autonomic hyperactivity, bowel hyper-motility, temperature instability, pain and a sense of impending doom. Withdrawal is generally not associated with seizures in adults but is characterized by extreme agitation, aggression and irritability. Signs: Opioid withdrawal involves a constellation of symptoms. Typically withdrawal presents concurrently as several of the following: • psychological symptoms (e.g., cravings, insomnia, fatigue) • flu-like physical symptoms (e.g., myalgias, chills, nausea, diarrhea). Objective signs of withdrawal are usually not present except on sudden cessation of high doses of opioids such as oral oxycodone formulations, heroin or parenteral hydromorphone. Objective signs include: • agitation, restlessness • tearing, yawning, runny nose • vomiting • sweating, piloerection (goose bumps) • tachycardia, hypertension. Physical symptoms begin from six to 24 hours after last use, peak in two to three days and largely resolve in five to 10 days. -

What Is Skin? Skin Has a Big Job
Student Activity Sheet Name: What Is Skin? Skin has a big job. It’s your body’s largest organ. It protects your body. It keeps germs and dirt from getting inside your body. It keeps you cool and warm. And it has nerves that help you feel. There are millions of skin cells on your body. Did you know that you lose thousands and thousands of skin cells every day? The good news is that your skin grows back quickly. tUVB (rays that cause sunburn on the skin’s surface) Exploring Skin’s Layers tUVA (rays that penetrate deep into the dermis) Skin has three layers. 1. Epidermis—New skin cells are made here. It is also where Epidermis melanin is made. Melanin is (Top Layer) what gives skin its color. 2. Dermis—It has nerves that Dermis help you feel sensations. It’s (Middle Layer) also where sweat, oil, and goose bumps are made. It helps bring blood to your Subcutaneous skin. It is also where hairs start. (Bottom Layer) 3. Subcutaneous—It connects the dermis to your muscles and bones and stores fat to help protect them from bumps and falls. It helps your blood vessels and nerve cells reach the rest of your body. This layer helps keep your body from getting too hot or cold. Protecting Your Skin Whenever you go outside, your skin • Wear a wide-brimmed hat. It will is exposed to the sun. The sun has protect your face, head, ears, ultraviolet (UV) rays that can burn and and neck more than a baseball cap. -

Nothing but Skin and Bone F
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2006 Nothing but skin and bone F. Patrick Ross Washington University School of Medicine in St. Louis Angela M. Christiano Columbia University College of Physicians and Surgeons Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Ross, F. Patrick and Christiano, Angela M., ,"Nothing but skin and bone." The ourJ nal of Clinical Investigation.,. 1140-1149. (2006). https://digitalcommons.wustl.edu/open_access_pubs/1558 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Downloaded on August 5, 2013. The Journal of Clinical Investigation. More information at www.jci.org/articles/view/28605 Review series introduction Nothing but skin and bone F. Patrick Ross1 and Angela M. Christiano2 1Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri, USA. 2Departments of Dermatology and Genetics and Development, Columbia University College of Physicians and Surgeons, New York, New York, USA. Skin and bone — what comes to mind at hearing this phrase? While certainly a metaphor for disease, it also defines two very different tissues, one a flexible and contiguous outer covering, the other a morphologically diverse hard tissue distributed at over 200 sites in the body. As the accompanying series of Reviews highlights, these tissues are indeed diverse, but there are also surprising similarities. Skin is the interface between the internal organs and the environment, and as such plays a crucial role in the body’s defense mechanism. -

Chapter 6 – the Integumentary System
Chapter 6 – The Integumentary System I. The Skin and Subcutaneous Tissue: The skin is the body’s largest organ, accounting for 15% of the body weight of adults. Please see the terms for Figure 6.1 on page ________. Be sure to recognize the picture for your exam. The skin consists of two layers: Epidermis (outer layer made of stratified squamous epithelium) and dermis (deeper layer made of connective tissue). Hypodermis is a layer of subcutaneous fat that lies below the two layers of the skin. Skin is classified as thick or thin based on the relative thickness of the epidermis. Thick skin covers the palms, soles, and surfaces of the fingers and toes. It has a thick stratum corneum, a layer of dead cells. Thick skin has sweat glands, but no hair follicles or sebaceous (oil) glands. Thin skin has a thin stratum corneum and possesses sweat glands, hair follicles, and sebaceous glands. A. Functions of the Skin 1. Resistance to trauma and infection – epidermal cells contain a tough protein called keratin that gives the skin durability. Also, skin is usually dry and slightly acidic to protect the body from bacteria & fungal infections. 2. Other barrier functions – it keeps out excess water and keeps the body from losing excess water. The epidermis also blocks harmful UV (ultraviolet) rays, thereby keeping cancer-causing radiation from deeper tissues. The skin keeps out harmful chemicals, but is permeable to several drugs and poisons. 3. Vitamin D synthesis – Vitamin D is needed for bone development and maintenance. 4. Sensation – The skin is equipped with many nerve endings that react to heat, cold, touch, texture, pressure, vibration and tissue injury. -

Université Paris Xi Faculté De Médecine Paris-Sud
UNIVERSITÉ PARIS XI FACULTÉ DE MÉDECINE PARIS-SUD Année 2010 N°attribué par la bibliothèque THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE PARIS XI Champ disciplinaire : Médecine Ecole Doctorale de rattachement : ED418 Cancérologie, Biologie, Médecine,Santé Présentée et soutenue publiquement par L’Emira Ghida HARFOUCHE Le 12 février 2010 Titre: FIBROBLAST GROWTH FACTOR 2 AND DNA REPAIR INVOLVEMENT IN THE KERATINOCYTE STEM CELL RESPONSE TO IONIZING RADIATION Directrice de thèse: MARTIN Michèle JURY Pr. BOURHIS Jean Président Dr. DOUKY Thierry Rapporteur Pr. LAMARTINE Jérôme Rapporteur Dr. BRETON Lionel Examinateur Dr. MOYAL Elisabeth Examinateur Dr. MARTIN Michèle Directrice de thèse ACKNOWLEDGEMENTS First of all, I would like to thank my thesis advisor, Dr. Michele Martin, for her guidance and her expertise in stem cell radiobiology. I appreciate her enthusiasm and encouragement during the course of the study. I have learned a lot and have grown a lot through all the experiences in her lab, not only as a scientist but also as a person. I would like to acknowledge my thesis committee Dr. Thierry Douky and Pr. Jérôme Lamartine for accepting to be my “rapporteurs”, for evaluating my work, for carefully reading this thesis and giving me helpful instructions and for being part of the jury members, along with Pr. Jean Bourhis, Dr. Lionel Breton, and Dr. Elisabeth Moyal. Their contributions to the judgment of this work and to this thesis as readers are very much appreciated. I would like to thank Dr. Nicolas Fortunel for his encouragement, invaluable advices and discussions that made me leap up one more step as a scientist. -

What Is Skin? Skin Has a Big Job
Student Activity Sheet Name: What Is Skin? Skin has a big job. It’s your body’s largest organ. It protects your body. It keeps germs and dirt from getting inside your body. It keeps you cool and warm. And it has nerves that help you feel. There are millions of skin cells on your body. Did you know that you lose thousands and thousands of skin cells every day? The good news is that your skin grows back quickly. tUVB (rays that cause sunburn on the skin’s surface) Exploring Skin’s Layers tUVA (rays that penetrate deep into the dermis) Skin has three layers. 1. Epidermis—New skin cells are made here. It is also where Epidermis melanin is made. Melanin is (Top Layer) what gives skin its color. 2. Dermis—It has nerves that Dermis help you feel sensations. It’s (Middle Layer) also where sweat, oil, and goose bumps are made. It helps bring blood to your Subcutaneous skin. It is also where hairs start. (Bottom Layer) 3. Subcutaneous—It connects the dermis to your muscles and bones and stores fat to help protect them from bumps and falls. It helps your blood vessels and nerve cells reach the rest of your body. This layer helps keep your body from getting too hot or cold. Protecting Your Skin Whenever you go outside, your skin • Wear a wide-brimmed hat. It will is exposed to the sun. The sun has protect your face, head, ears, ultraviolet (UV) rays that can burn and and neck more than a baseball cap. -
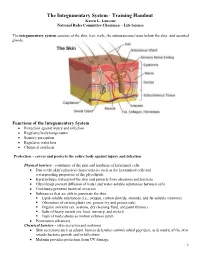
The Integumentary System - Training Handout Karen L
The Integumentary System - Training Handout Karen L. Lancour National Rules Committee Chairman – Life Science The integumentary system consists of the skin, hair, nails, the subcutaneous tissue below the skin, and assorted glands. Functions of the Integumentary System Protection against injury and infection Regulates body temperature Sensory perception Regulates water loss Chemical synthesis Protection – covers and protects the entire body against injury and infection Physical barriers - continuity of the skin and hardness of keratinzed cells Due to the skin’s physical characteristics such as the keratinized cells and waterproofing properties of the glycolipids. Keratin helps waterproof the skin and protects from abrasions and bacteria Glycolipids prevent diffusion of water and water-soluble substances between cells Continuity prevents bacterial invasion Substances that are able to penetrate the skin: . Lipid-soluble substances (i.e., oxygen, carbon dioxide, steroids, and fat-soluble vitamins) . Oleoresins of certain plants (ex. poison ivy and poison oak) . Organic solvents (ex. acetone, dry cleaning fluid, and paint thinner) . Salts of heavy metals (ex. lead, mercury, and nickel) . Topical medications as motion sickness patch Penetration enhancers Chemical barriers - (skin secretion and melanin) Skin secretions such as sebum, human defensins (antimicrobial peptides), acid mantle of the skin retards bacteria growth and/or kills them Melanin provides protection from UV damage 1 Skin secretions (acid mantle) Low pH and sebum slow bacterial growth on skin surface Human defensin – natural antibiotic Cathelicidins – proteins that prevent Strep A infection in wounded skin Melanin – chemical pigment that prevents UV damage Biological Barriers Langerhans’ cells, macrophages, and DNA Langerhans’ cells in epidermis present antigens to lymphocytes Dermal macrophages (2nd line of defense) – attack bacteria and viruses that have penetrated the epidermis Langerhan’s cells and macrophages present in the skin helps activate the body’s immune system.