Arceuthobium Spp.) in the Southwest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Forest Action Plan 2015 Status Report and Addendum
Arizona Forest Action Plan 2015 Status Report and Addendum A report on the strategic plan to address forest-related conditions, trends, threats, and opportunities as identified in the 2010 Arizona Forest Resource Assessment and Strategy. November 20, 2015 Arizona State Forestry Acknowledgements: Arizona State Forestry would like to thank the USDA Forest Service for their ongoing support of cooperative forestry and fire programs in the State of Arizona, and for specific funding to support creation of this report. We would also like to thank the many individuals and organizations who contributed to drafting the original 2010 Forest Resource Assessment and Resource Strategy (Arizona Forest Action Plan) and to the numerous organizations and individuals who provided input for this 2015 status report and addendum. Special thanks go to Arizona State Forestry staff who graciously contributed many hours to collect information and data from partner organizations – and to writing, editing, and proofreading this document. Jeff Whitney Arizona State Forester Granite Mountain Hotshots Memorial On the second anniversary of the Yarnell Hill Fire, the State of Arizona purchased 320 acres of land near the site where the 19 Granite Mountain Hotshots sacrificed their lives while battling one of the most devastating fires in Arizona’s history. This site is now the Granite Mountain Hotshots Memorial State Park. “This site will serve as a lasting memorial to the brave hotshots who gave their lives to protect their community,” said Governor Ducey. “While we can never truly repay our debt to these heroes, we can – and should – honor them every day. Arizona is proud to offer the public a space where we can pay tribute to them, their families and all of our firefighters and first responders for generations to come.” Arizona Forest Action Plan – 2015 Status Report and Addendum Background Contents The 2010 Forest Action Plan The development of Arizona’s Forest Resource Assessment and Strategy (now known as Arizona’s “Forest Action Plan”) was prompted by federal legislative requirements. -

Trace and Minor Element Analysis of Obsidian From
r r TRACE AND MINOR ELEMENT ANALYSIS OF OBSIDIAN r FROM THE SAN FRANCISCO VOLCANIC FIELD r USING X-RAY FLUORESCENCE r r [ r A Thesis Presented to the Graduate Faculty F' ! 1. Northern Arizona University r ~ l rL In Partial Fulfillment r of the Requirements for the Degree r Master of Science r ' r r \. by r Suzanne C. Sanders L April 1981 _[r r t f l I. r l l t I ABSTRACT . r l f ! f r I Obsidian from eight locations in the San Franciscan volcanic field in northern Arizona were analyzed for 20 minor and trace elements r using x-ray fluorescence analysis. The intensity ratios relative to iron were statistically analyzed r and the trace and minor element patterns established. The obsidian rL outcrops clustered into four well defined groups consisting of two localities apiece: Government Mountain/Obsidian Tank, Slate Mountain/ r Kendrick Peak, Robinson Crater/O'Leary Peak, and RS Hill/Spring Valley. r Each of the four distinct groups was treated individually to refine the separation between the similar sites. Classification function coeffi r cients were calculated for each locality, then these were used to identify the source of thirteen obsidian artifacts recovered from a Northern r Arizona archaeological site. r r r r r r r r l r ..r r r r I CONTENTS r t Page LIST OF TABLES .. iii r LIST OF FIGURES . v r Chapter 1. INTRODUCTION . 1 r, 2. SAMPLE COLLECTION 7 3. METHODS AND MATERIALS 12 r 4. ANALYSIS OF THE DATA .. 17 r 5. -

KAIBAB DEER HERD MUST BE REDUCED IMMEDIATELY -- October 13,1924
U.S.DEPARTMENT OFAGRICULTURE Office of the Secretary , Pra serviq ” _, RELEASED FOR PUBLICATION, MONDAYMORNING, OCTOBER13, 19+: -9 KAIBAB DEER HERD MUST BE REDUCEDIXMEDIATELY Immediate reduction of the deer herd on the Kaibab National Forest in northern Arizona is strongly urged by the special committee appointed by the Secretary of Agriculture to study and report on the conditions existing on the Grand Canyon Game Preserve, announces the Forest Service, United States Depart- ment of Agricu&are. ! The special committee is composed of John B. Burnham, chairman, repre- senting the American Game Protective Association; Heyward Cutting, of tho Boone and Crockett Club; T. Gilbert Pearson,, of the Audubon Society and the National Parks Association; and T. W. Tomlinson, of the American National Livestock Association. This ccmmittee has made its report to the Secretary of Agriculture following a personal inspection of the Kaibab Plateau on which the Grand Canyon &me Preserve was estabZ.shed in 1906 by President Roosevelt. This area also forms part of the Kaibab Nakional Forest and is under the supervision Of the Forest Servi&. _ . , Upwards of 30,000 head of mule deer are now on the Kaibab Plateau, according to the report of the committee. This is fully twice as many deer as the vegetation can support and the entire herd is in imdnent danger of extinction through starvation unless reduced to a safety number. Moreover, the condition of That forage is still to be found on the ' arsa is far below nornal and several years will be required to grow new forage crops before the region can support more than 15,009 head of deer'in addition to the scattering amal 1 herds of domestic livest.ock owned by settlers living in and around the Kaibab Forest. -

Summits on the Air – ARM for the USA (W7A
Summits on the Air – ARM for the U.S.A (W7A - Arizona) Summits on the Air U.S.A. (W7A - Arizona) Association Reference Manual Document Reference S53.1 Issue number 5.0 Date of issue 31-October 2020 Participation start date 01-Aug 2010 Authorized Date: 31-October 2020 Association Manager Pete Scola, WA7JTM Summits-on-the-Air an original concept by G3WGV and developed with G3CWI Notice “Summits on the Air” SOTA and the SOTA logo are trademarks of the Programme. This document is copyright of the Programme. All other trademarks and copyrights referenced herein are acknowledged. Document S53.1 Page 1 of 15 Summits on the Air – ARM for the U.S.A (W7A - Arizona) TABLE OF CONTENTS CHANGE CONTROL....................................................................................................................................... 3 DISCLAIMER................................................................................................................................................. 4 1 ASSOCIATION REFERENCE DATA ........................................................................................................... 5 1.1 Program Derivation ...................................................................................................................................................................................... 6 1.2 General Information ..................................................................................................................................................................................... 6 1.3 Final Ascent -

San Francisco Mountains Forest Heserve, Arizona
Professional Paper No. 22 Series H, Forestry, 7 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES D. WALCOTT, DIRECTOR \ FOREST CONDITIONS lN 'fHE- SAN FRANCISCO MOUNTAINS FOREST HESERVE, ARIZONA BY JOHN B. LEIBERG, THEODORE F. RIXON, AND ARTHUR DODWELL WITH AN INTRODUCTION BY F. G. PLUMMER ·wASHINGTON G 0 Y E R N 1\I E N T I' HI N T IN G 0 ];' F I C E 1904 CONT .. ENTS. Page.. Letter of transmittaL_. __._._. ____-_._._._ .. _._ .. _.. _. ______ . __ _._._._- __._. ________ ._· __________ .~ __ . ____ . __ .___ 9 Introdnetion.·.-_-_. ______________ ._._._._._._._. ___ .. _______ .___ -_._. __ . ___________ . ____ . _. _________ ---- _ ___ 11 Boundaries·. ___ .----- ___ .·.·-·-·-·-·-·-·-_._. __ . __________ ._ .. _.._._. ______ . _______________ . _____ .___ 11 Surface features ___ . _ _- ..·: ______ ._._._ ..·.- ___ .· _ _. _ _. __ . _ _. ___________ .: ___________ . ________ ._______ 13 Soil·. _. _: _. _.. __ _. .. _ . _. __ .. __. .· ..· .... _. _. _..: ____ _. __. __ _. .· __.. __ . ___ .. __ . __ . __ . __ .. _____ . ___ .. 15 Drainage_._. _ _. ___ . _____________________ . __________ _.:. _ _. ________ . ____ . ___ ------_________ 15 Forest and womllan<.l .· __ . __ . __ . __ . __ . _____ ·- : _.. ·_____ . ___________ . ____ .· __ . _____ . _....... _ _ 17 Zones or types of arborescent growth_ . _.·. _. __.: _. __ . _: __. ... ____ . _______ . __ . __ . _.. _.. ___ . _ 18 Aspect and character of timber belts ____ . -
Grand Canyon
U.S. Department of the Interior Geologic Investigations Series I–2688 14 Version 1.0 4 U.S. Geological Survey 167.5 1 BIG SPRINGS CORRELATION OF MAP UNITS LIST OF MAP UNITS 4 Pt Ph Pamphlet accompanies map .5 Ph SURFICIAL DEPOSITS Pk SURFICIAL DEPOSITS SUPAI MONOCLINE Pk Qr Holocene Qr Colorado River gravel deposits (Holocene) Qsb FAULT CRAZY JUG Pt Qtg Qa Qt Ql Pk Pt Ph MONOCLINE MONOCLINE 18 QUATERNARY Geologic Map of the Pleistocene Qtg Terrace gravel deposits (Holocene and Pleistocene) Pc Pk Pe 103.5 14 Qa Alluvial deposits (Holocene and Pleistocene) Pt Pc VOLCANIC ROCKS 45.5 SINYALA Qti Qi TAPEATS FAULT 7 Qhp Qsp Qt Travertine deposits (Holocene and Pleistocene) Grand Canyon ၧ DE MOTTE FAULT Pc Qtp M u Pt Pleistocene QUATERNARY Pc Qp Pe Qtb Qhb Qsb Ql Landslide deposits (Holocene and Pleistocene) Qsb 1 Qhp Ph 7 BIG SPRINGS FAULT ′ × ′ 2 VOLCANIC DEPOSITS Dtb Pk PALEOZOIC SEDIMENTARY ROCKS 30 60 Quadrangle, Mr Pc 61 Quaternary basalts (Pleistocene) Unconformity Qsp 49 Pk 6 MUAV FAULT Qhb Pt Lower Tuckup Canyon Basalt (Pleistocene) ၣm TRIASSIC 12 Triassic Qsb Ph Pk Mr Qti Intrusive dikes Coconino and Mohave Counties, Pe 4.5 7 Unconformity 2 3 Pc Qtp Pyroclastic deposits Mr 0.5 1.5 Mၧu EAST KAIBAB MONOCLINE Pk 24.5 Ph 1 222 Qtb Basalt flow Northwestern Arizona FISHTAIL FAULT 1.5 Pt Unconformity Dtb Pc Basalt of Hancock Knolls (Pleistocene) Pe Pe Mၧu Mr Pc Pk Pk Pk NOBLE Pt Qhp Qhb 1 Mၧu Pyroclastic deposits Qhp 5 Pe Pt FAULT Pc Ms 12 Pc 12 10.5 Lower Qhb Basalt flows 1 9 1 0.5 PERMIAN By George H. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Arizona's Wildlife Linkages Assessment
ARIZONAARIZONA’’SS WILDLIFEWILDLIFE LINKAGESLINKAGES ASSESSMENTASSESSMENT Workgroup Prepared by: The Arizona Wildlife Linkages ARIZONA’S WILDLIFE LINKAGES ASSESSMENT 2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT Arizona’s Wildlife Linkages Assessment Prepared by: The Arizona Wildlife Linkages Workgroup Siobhan E. Nordhaugen, Arizona Department of Transportation, Natural Resources Management Group Evelyn Erlandsen, Arizona Game and Fish Department, Habitat Branch Paul Beier, Northern Arizona University, School of Forestry Bruce D. Eilerts, Arizona Department of Transportation, Natural Resources Management Group Ray Schweinsburg, Arizona Game and Fish Department, Research Branch Terry Brennan, USDA Forest Service, Tonto National Forest Ted Cordery, Bureau of Land Management Norris Dodd, Arizona Game and Fish Department, Research Branch Melissa Maiefski, Arizona Department of Transportation, Environmental Planning Group Janice Przybyl, The Sky Island Alliance Steve Thomas, Federal Highway Administration Kim Vacariu, The Wildlands Project Stuart Wells, US Fish and Wildlife Service 2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT First Printing Date: December, 2006 Copyright © 2006 The Arizona Wildlife Linkages Workgroup Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written consent from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written consent of the copyright holder. Additional copies may be obtained by submitting a request to: The Arizona Wildlife Linkages Workgroup E-mail: [email protected] 2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT The Arizona Wildlife Linkages Workgroup Mission Statement “To identify and promote wildlife habitat connectivity using a collaborative, science based effort to provide safe passage for people and wildlife” 2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT Primary Contacts: Bruce D. -

Kaibab National Forest
United States Department of Agriculture Kaibab National Forest Forest Service Southwestern Potential Wilderness Area Region September 2013 Evaluation Report The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means of communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TTY). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, SW, Washington, DC 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TTY). USDA is an equal opportunity provider and employer. Cover photo: Kanab Creek Wilderness Kaibab National Forest Potential Wilderness Area Evaluation Report Table of Contents Introduction ................................................................................................................................................. 1 Inventory of Potential Wilderness Areas .................................................................................................. 2 Evaluation of Potential Wilderness Areas ............................................................................................... -

MTB Participant Manual
MTB Participant Manual Flagstaff to Grand Canyon Stagecoach Line 100-Mile Mountain Bike Race ____________________________________________ Flagstaff, Arizona September 19, 2020 6AM ____________________________________________ Presented by Babbitt Ranches Flagstaff to Grand Canyon 100 Table of Contents Race Management 3 Sponsors 4 Brief Course Description 5 Qualifying 5 Start Times 5 Time Limits & Cut-Off Requirements 5 Race Day Parking 6 Post-Race Transportation 6 Flagstaff, AZ 6 Tusayan, AZ 7 Travel 7 Accommodations 7 Race Weekend Weather 8 Our Partners 9 Awards 12 Race Weekend Agenda 13 Course Marking 14 Aid Stations 14 Race Mechanics 14 Drop Bags 15 Recommended Gear 16 Last Minute Supplies 16 Social Media 16 Aid Station Matrix: Distances, Relay Exchanges, and Services 17 Biker Rules 17 Crew Rules 19 Aid Station Driving Directions 21 Recommended Purchases 24 Detailed Course Description 25 ©2020 The Flagstaff to Grand Canyon Stagecoach Line races are conducted under special use permit of the Coconino and Kaibab National Forests. 2 Flagstaff to Grand Canyon 100 Race Management Race Director Ian Torrence Co-Race Director Emily Torrence Mountain Bike Co-Race Director Dana Ernst Medical Directors Eric True & Scott Bajer Coconino County Sheriff’s SAR Coordinator Bart Thompson Coconino Amateur Radio Club Coordinator Joe Hobart Timing & Tracking Run Flagstaff and UltraLive.net 3 Brief Event Description This mountain bike event begins a few miles north of Flagstaff, Arizona, near the intersection of Snowbowl Road and Route 180, and finishes in Tusayan, Arizona, the entrance of Grand Canyon National Park. A majority of the Stagecoach course follows the Arizona Trail and the historic Flagstaff to Grand Canyon Stagecoach Line route used by adventure seeking tourists between 1897 and 1901. -

A Regional Climatology of Monsoonal Precipitation in the Southwestern United States Using TRMM
310 JOURNAL OF HYDROMETEOROLOGY VOLUME 13 A Regional Climatology of Monsoonal Precipitation in the Southwestern United States Using TRMM CHRISTINA L. WALL,EDWARD J. ZIPSER, AND CHUNTAO LIU University of Utah, Salt Lake City, Utah (Manuscript received 17 March 2011, in final form 22 June 2011) ABSTRACT Using 13 yr of data from the Tropical Rainfall Measuring Mission (TRMM) satellite, a regional climatology of monsoonal precipitation is created for portions of the southwest United States. The climatology created using precipitation features defined from the TRMM precipitation radar (PR) shows that the population of features includes a large number of small, weak features that do not produce much rain and are very shallow. A lesser percentage of large, stronger features contributes most of the region’s rainfall. Dividing the features into categories based on the median values of volumetric rainfall and maximum height of the 30-dBZ echo is a useful way to visualize the population of features, and the categories selected reflect the life cycle of monsoonal convection. An examination of the top rain-producing features at different elevations reveals that extreme features tend to occur at lower elevations later in the day. A comparison with the region studied in the North American Monsoon Experiment (NAME) shows that similar diurnal patterns occur in the Sierra Madre Occidental region of Mexico. The population of precipitation features in both regions is similar, with the NAME region producing slightly larger precipitation systems on average than the southwest United States. Both regions on occasion demonstrate the pattern of convection initiating at high elevations and moving downslope while growing upscale through the afternoon and evening; however, there are also days on which convection remains over the high terrain. -
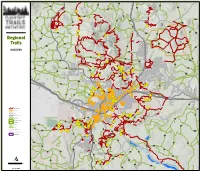
Basemap – Dot Exercise
ak k Pe 419 ric nd Kendrick Park Ke 773 546 760 Kendrick Park 6005 Wildlife 545B O'Leary Peak 418 W h i te Ho rs e H ills O'Leary 418 Lookout 418 193 erffer H Bear Hochd ills Jaw 9124N Sunset Crater 180 552 552 National Monument Abineau 151 Lava's Edge 9140K Nordic Village Waterline Lava Sunset Crater Bismarck Flow Lake Lockett 420 Meadow Lenox Crater Rees Aubineau Peak Peak Fern 244 Mtn Humphreys 794 H Peak 244A a 245 r 9141U t P 776 r a i r i e Humphreys Cinder Lake Inner Basin Regional 9004K Aspen Nature Doyle Peak Brandis Agassiz Peak C 9004L Waterline 9125G i n d e r Trails L Fremont a k Peak e 9008L s 420 Fernwood M a Timberline n a 244B g e Schultz m Peak e n t A r e Weatherford a Deer SUCCESS Veit Kachina 6064D Hill Spring 9123Q 151 Arizona 522 222 244 Secret 743 222B GT 9122P Newham 420 556B Secret Rocky 556 222A Moto Old Caves Sunset 511A Little Little Bear Elden Arizona Little Elden Mtn Wing Mtn 511 171 222 Moto Fort 164B Valley Schultz ills 519 Creek e H Brookbank ak 89 y L Dr Doney Park 557 9121Q Upper Sandy Oldham Heart Seep Chimney 180 Flue Christmas Rocky Tree Ridge Fatmans Loop 668D Elden Oldham Mt Elden Lookout Bellemont Tom Moody T 518 Picture u r Canyon k e A-1 Mtn Preserve y Don H il Weaver ls 510B Pipeline Buffalo Cosnino Park 40 Arizona Observatory Mesa Winona 518 515 506 Thorpe Observatory Park Sunnyside Mesa Continental Navajo Army Natural Area Depot Anasazi 40 Downtown Campbell Mesa Sinagua Walnut Meadows 745 Arizona Little America Recreational trails 764 FUTS trails NAU 40 Arizona on ny C a lnut Street Walnut Canyon Wa Dry Lake Loop National Rim Monument 82 Highway/major road Island Charles O.