Acute Liver Failure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Abnormal Liver Chemistries
ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries Paul Y. Kwo, MD, FACG, FAASLD1, Stanley M. Cohen, MD, FACG, FAASLD2, and Joseph K. Lim, MD, FACG, FAASLD3 1Division of Gastroenterology/Hepatology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California, USA; 2Digestive Health Institute, University Hospitals Cleveland Medical Center and Division of Gastroenterology and Liver Disease, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA; 3Yale Viral Hepatitis Program, Yale University School of Medicine, New Haven, Connecticut, USA. Am J Gastroenterol 2017; 112:18–35; doi:10.1038/ajg.2016.517; published online 20 December 2016 Abstract Clinicians are required to assess abnormal liver chemistries on a daily basis. The most common liver chemistries ordered are serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and bilirubin. These tests should be termed liver chemistries or liver tests. Hepatocellular injury is defined as disproportionate elevation of AST and ALT levels compared with alkaline phosphatase levels. Cholestatic injury is defined as disproportionate elevation of alkaline phosphatase level as compared with AST and ALT levels. The majority of bilirubin circulates as unconjugated bilirubin and an elevated conjugated bilirubin implies hepatocellular disease or cholestasis. Multiple studies have demonstrated that the presence of an elevated ALT has been associated with increased liver-related mortality. A true healthy normal ALT level ranges from 29 to 33 IU/l for males, 19 to 25 IU/l for females and levels above this should be assessed. The degree of elevation of ALT and or AST in the clinical setting helps guide the evaluation. -

A Drug-Induced Cholestatic Pattern
Review articles Hepatotoxicity: A Drug-Induced Cholestatic Pattern Laura Morales M.,1 Natalia Vélez L.,1 Octavio Germán Muñoz M., MD.2 1 Medical Student in the Faculty of Medicine and Abstract the Gastrohepatology Group at the Universidad de Antioquia in Medellín, Colombia Although drug induced liver disease is a rare condition, it explains 40% to 50% of all cases of acute liver 2 Internist and Hepatologist at the Hospital Pablo failure. In 20% to 40% of the cases, the pattern is cholestatic and is caused by inhibition of the transporters Tobon Uribe and in the Gastrohepatology Group at that regulate bile synthesis. This reduction in activity is directly or indirectly mediated by drugs and their me- the Universidad de Antioquia in Medellín, Colombia tabolites and/or by genetic polymorphisms and other risk factors of the patient. Its manifestations range from ......................................... biochemical alterations in the absence of symptoms to acute liver failure and chronic liver damage. Received: 30-01-15 Although there is no absolute test or marker for diagnosis of this disease, scales and algorithms have Accepted: 26-01-16 been developed to assess the likelihood of cholestatic drug induced liver disease. Other types of evidence are not routinely used because of their complexity and cost. Diagnosis is primarily based on exclusion using circumstantial evidence. Cholestatic drug induced liver disease has better overall survival rates than other patters, but there are higher risks of developing chronic liver disease. In most cases, the patient’s condition improves when the drug responsible for the damage is removed. Hemodialysis and transplantation should be considered only for selected cases. -

Acute Liver Failure J G O’Grady
148 Postgrad Med J: first published as 10.1136/pgmj.2004.026005 on 4 March 2005. Downloaded from REVIEW Acute liver failure J G O’Grady ............................................................................................................................... Postgrad Med J 2005;81:148–154. doi: 10.1136/pgmj.2004.026005 Acute liver failure is a complex multisystemic illness that account for most cases, but a significant number of patients have no definable cause and are evolves quickly after a catastrophic insult to the liver classified as seronegative or of being of indeter- leading to the development of encephalopathy. The minate aetiology. Paracetamol is the commonest underlying aetiology and the pace of progression strongly cause in the UK and USA.2 Idiosyncratic reac- tions comprise another important group. influence the clinical course. The commonest causes are paracetamol, idiosyncratic drug reactions, hepatitis B, and Viral seronegative hepatitis. The optimal care is multidisciplinary ALF is an uncommon complication of viral and up to half of the cases receive liver transplants, with hepatitis, occurring in 0.2%–4% of cases depend- ing on the underlying aetiology.3 The risk is survival rates around 75%–90%. Artificial liver support lowest with hepatitis A, but it increases with the devices remain unproven in efficacy in acute liver failure. age at time of exposure. Hepatitis B can be associated with ALF through a number of ........................................................................... scenarios (table 2). The commonest are de novo infection and spontaneous surges in viral repli- cation, while the incidence of the delta virus cute liver failure (ALF) is a complex infection seems to be decreasing rapidly. multisystemic illness that evolves after a Vaccination should reduce the incidence of Acatastrophic insult to the liver manifesting hepatitis A and B, while antiviral drugs should in the development of a coagulopathy and ameliorate replication of hepatitis B. -

Management of Autoimmune Liver Diseases After Liver Transplantation
Review Management of Autoimmune Liver Diseases after Liver Transplantation Romelia Barba Bernal 1,† , Esli Medina-Morales 1,† , Daniela Goyes 2 , Vilas Patwardhan 1 and Alan Bonder 1,* 1 Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA; [email protected] (R.B.B.); [email protected] (E.M.-M.); [email protected] (V.P.) 2 Department of Medicine, Loyola Medicine—MacNeal Hospital, Berwyn, IL 60402, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-617-632-1070 † These authors contributed equally to this project. Abstract: Autoimmune liver diseases are characterized by immune-mediated inflammation and even- tual destruction of the hepatocytes and the biliary epithelial cells. They can progress to irreversible liver damage requiring liver transplantation. The post-liver transplant goals of treatment include improving the recipient’s survival, preventing liver graft-failure, and decreasing the recurrence of the disease. The keystone in post-liver transplant management for autoimmune liver diseases relies on identifying which would be the most appropriate immunosuppressive maintenance therapy. The combination of a steroid and a calcineurin inhibitor is the current immunosuppressive regimen of choice for autoimmune hepatitis. A gradual withdrawal of glucocorticoids is also recommended. Citation: Barba Bernal, R.; On the other hand, ursodeoxycholic acid should be initiated soon after liver transplant to prevent Medina-Morales, E.; Goyes, D.; recurrence and improve graft and patient survival in primary biliary cholangitis recipients. Unlike the Patwardhan, V.; Bonder, A. Management of Autoimmune Liver previously mentioned autoimmune diseases, there are not immunosuppressive or disease-modifying Diseases after Liver Transplantation. -

Spontaneous Bacterial Peritonitis: Recent Guidelines and Beyond R Wiest,1 a Krag,2 a Gerbes3
Downloaded from gut.bmj.com on May 31, 2012 - Published by group.bmj.com Recent advances in clinical practice Spontaneous bacterial peritonitis: recent guidelines and beyond R Wiest,1 A Krag,2 A Gerbes3 1Department for visceral surgery INTRODUCTION prognosis in various cohorts of patients with SBP and medicine, University Spontaneous bacterial peritonitis (SBP) is the most are summarised in figure 1 and include age,16 20 Hospital Bern, Switzerland 18 20 23 16 18 2 frequent and life-threatening infection in patients Child score, intensive care, nosocomial Department of 18 24 25 Gastroenterology, Hvidovre with liver cirrhosis requiring prompt recognition origin, hepatic encephalopathy, elevated 26 University Hospital, and treatment. It is defined by the presence of >250 serum creatinine and bilirubin, lack of infection Copenhagen, Denmark polymorphonuclear cells (PMN)/mm3 in ascites in resolution/need to escalate treatment and culture 3 e Klinikum of the University of the absence of an intra-abdominal source of infec- positivity27 29 as well as the presence of bacter- Munich, Munich, Germany tion or malignancy. In this review we discuss the aemia30 and CARD15/NOD2 variants as a genetic fl 31 Correspondence to current opinions re ected by recent guidelines risk factor. It is important to stress in this context Professor Dr Reiner Wiest, (American Association for the Study of Liver that the only factors that are modifiable in this Department for visceral surgery Diseases, European Association for the Study of the scenario are timely diagnosis and effective first-line and medicine, University Liver, Deutsche Gesellschaft für Verdauungs- und treatment. Hospital Bern, 3010 Bern, 1e4 Switzerland; Stoffwechselkrankheiten), with particular focus [email protected] on controversial issues as well as open questions Bacterial translocation (BT) and pathophysiology that need to be addressed in the future. -
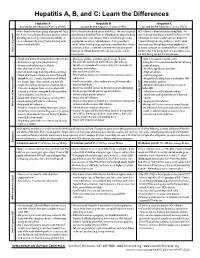
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Diagnosis and Management of Primary Biliary Cholangitis Ticle
REVIEW ArtICLE 1 see related editorial on page x Diagnosis and Management of Primary Biliary Cholangitis TICLE R Zobair M. Younossi, MD, MPH, FACG, AGAF, FAASLD1, David Bernstein, MD, FAASLD, FACG, AGAF, FACP2, Mitchell L. Shifman, MD3, Paul Kwo, MD4, W. Ray Kim, MD5, Kris V. Kowdley, MD6 and Ira M. Jacobson, MD7 Primary biliary cholangitis (PBC) is a chronic, cholestatic, autoimmune disease with a variable progressive course. PBC can cause debilitating symptoms including fatigue and pruritus and, if left untreated, is associated with a high risk of cirrhosis and related complications, liver failure, and death. Recent changes to the PBC landscape include a REVIEW A name change, updated guidelines for diagnosis and treatment as well as new treatment options that have recently become available. Practicing clinicians face many unanswered questions when managing PBC. To assist these healthcare providers in managing patients with PBC, the American College of Gastroenterology (ACG) Institute for Clinical Research & Education, in collaboration with the Chronic Liver Disease Foundation (CLDF), organized a panel of experts to evaluate and summarize the most current and relevant peer-reviewed literature regarding PBC. This, combined with the extensive experience and clinical expertise of this expert panel, led to the formation of this clinical guidance on the diagnosis and management of PBC. Am J Gastroenterol https://doi.org/10.1038/s41395-018-0390-3 INTRODUCTION addition, diagnosis and treatment guidelines are changing and a Primary biliary cholangitis (PBC) is a chronic, cholestatic, auto- number of guidelines have been updated [4, 5]. immune disease with a progressive course that may extend over Because of important changes in the PBC landscape, and a num- many decades. -

Managing Liver Failure D a Kelly
660 Postgrad Med J: first published as 10.1136/pmj.78.925.660 on 1 November 2002. Downloaded from BEST PRACTICE Managing liver failure D A Kelly ............................................................................................................................. Postgrad Med J 2002;78:660–667 Liver disease is rare in childhood, but important new Clinical presentation developments have altered the natural history and The clinical presentation depends on the aeti- ology of acute liver failure but the presentation outcome. It is important that clinicians are aware of may either be acute, within days, or prolonged to these diseases and their management. Acute liver failure 10 weeks if due to metabolic liver disease. The is most often due to viral hepatitis, paracetamol extent of jaundice and encephalopathy is variable in the early stages, but all children have coagu- overdose, or inherited metabolic liver disease. The lopathy. Encephalopathy is particularly difficult to clinical presentation includes jaundice, coagulopathy, diagnose in neonates. Vomiting and poor feeding and encephalopathy. Early diagnosis is necessary to are early signs, while irritability and reversal of day/night sleep patterns indicates more estab- prevent complications such as cerebral oedema, lished hepatic encephalopathy. In older children gastrointestinal bleeding, and renal failure. Early encephalopathy may present with aggressive supportive management, in particular intravenous behaviour or convulsions. N-acetylcysteine, may be effective but liver Neonatal acute liver failure transplantation is usually the definitive treatment and The commonest cause of acute liver failure in thus early referral to a specialist unit for liver neonates is septicaemia secondary to Escherichia coli, Staphylococcus aureus, or herpes simplex (box 1 transplantation is mandatory. Chronic liver failure may and table 1). -

Acute Liver Failure
Acute Liver Failure ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• When liver failure occurs in a previou ly healthy individual, it onset is often o rapid that it take experienced physicians by WILLIAM M. LEE surprise. Terms such a hyperacute or fulminant hepatic necrosis have been u ed to emphasize the rapidity and everity of the JULIE POLSON condition. The onset of acute liver failure is heralded by an altered mental state, often with agitation and confusion that may proceed to coma, and by evidence of coagulopathy [1 - 4]. The diagno is of acute liver failure require that these symptom develop in a patient without preexisting chronic liver disease. Although not all observers agree on precise definitions, it is generally accepted that onset of liver-related illness (usually, but not always, accompanied by jaundice) les than 8 weeks before patient presentation with altered mentation and coagulopathy fits the designation of acute liver failure. Development of these signs within a week of onset of liver disease and as much as 6 months from the first sign of illness is sometimes referred to as hyperacute and ubacute liver failure, respectively. An immediate challenge in managing sud1 patients is deter mination of the cause of acute liver failure in each case. Wherea certain factors, such a extent of encephalopathy and development of various complications, have an impact on outcome, the underlying etiology best determines the likeli hood of spontaneous survival or the need for urgent liver trans plantation [3,4]. The primary cause of acute liver failure vary in prevalence in different region of the world, and include drug toxicity and viral infections; there i also a large category of cases in which the pecific etiology cannot be determined. -

Guideline for the Evaluation of Cholestatic Jaundice
CLINICAL GUIDELINES Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition ÃRima Fawaz, yUlrich Baumann, zUdeme Ekong, §Bjo¨rn Fischler, jjNedim Hadzic, ôCara L. Mack, #Vale´rie A. McLin, ÃÃJean P. Molleston, yyEzequiel Neimark, zzVicky L. Ng, and §§Saul J. Karpen ABSTRACT Cholestatic jaundice in infancy affects approximately 1 in every 2500 term PREAMBLE infants and is infrequently recognized by primary providers in the setting of holestatic jaundice in infancy is an uncommon but poten- physiologic jaundice. Cholestatic jaundice is always pathologic and indicates tially serious problem that indicates hepatobiliary dysfunc- hepatobiliary dysfunction. Early detection by the primary care physician and tion.C Early detection of cholestatic jaundice by the primary care timely referrals to the pediatric gastroenterologist/hepatologist are important physician and timely, accurate diagnosis by the pediatric gastro- contributors to optimal treatment and prognosis. The most common causes of enterologist are important for successful treatment and an optimal cholestatic jaundice in the first months of life are biliary atresia (25%–40%) prognosis. The Cholestasis Guideline Committee consisted of 11 followed by an expanding list of monogenic disorders (25%), along with many members of 2 professional societies: the North American Society unknown or multifactorial (eg, parenteral nutrition-related) causes, each of for Gastroenterology, Hepatology and Nutrition, and the European which may have time-sensitive and distinct treatment plans. Thus, these Society for Gastroenterology, Hepatology and Nutrition. This guidelines can have an essential role for the evaluation of neonatal cholestasis committee has responded to a need in pediatrics and developed to optimize care. -

Sudden (Acute) Liver Failure
Customer Name, Street Address, City, State, Zip code Phone number, Alt. phone number, Fax number, e-mail address, web site Sudden (Acute) Liver Failure Basics OVERVIEW • Sudden (acute) damage to the liver tissue that is so severe that the liver is unable to function properly and meet the needs of the body • Sudden (acute) loss of more than 75% of functional liver tissue; occurs primarily because of sudden (acute), massive death of liver tissue (known as “hepatic necrosis”), which has a catastrophic effect on multiple organs in a previously healthy pet; may rapidly lead to death • The liver is the largest gland in the body; it has many functions, including production of bile (a fluid substance involved in digestion of fats); production of albumin (a protein in the plasma of the blood); and detoxification of drugs and other chemicals (such as ammonia) in the body SIGNALMENT/DESCRIPTION OF PET Species • Dogs • Cats • More common in dogs than in cats Breed Predilections • Breeds that appear to have increased likelihood of having long-term inflammation of the liver (known as “chronic hepatitis”) as compared to other breeds may have higher risk of sudden (acute) liver failure SIGNS/OBSERVED CHANGES IN THE PET • Sudden (acute) onset • Sluggishness (lethargy) • Decreased appetite (known as “inappetence”) • Vomiting • Small intestinal diarrhea—may be bloody • Increased thirst (known as “polydipsia”) and increased urination (known as “polyuria”) • Enlargement of the liver (known as “hepatomegaly”), with tenderness of the liver on feeling the abdomen -

COVID-19 and Liver Cirrhosis Important Information for Patients and Their Families
COVID-19 and Liver Cirrhosis Important Information for Patients and Their Families The American Association for the Study of Liver Diseases (AASLD) is committed to helping you understand coronavirus disease 2019 (COVID-19) infection and prevention in people with liver cirrhosis. What We Know Our understanding of COVID-19 in people with liver cirrhosis is evolving. When making decisions related to COVID-19 infections or prevention, having up-to-date information is critical. • Symptoms of COVID-19 infection include any of the following: fever, chills, drowsiness, cough, congestion or runny nose, difficulty breathing, fatigue, body aches, headache, sore throat, abdominal pain, nausea, vomiting, diarrhea, and loss of sense of taste or smell. • People with underlying cirrhosis of the liver are at a higher risk of developing severe COVID-19 illness and/or more problems from their existing liver disease if they get a COVID-19 infection, with prolonged hospitalization and increased mortality. These patients need to take careful precautions to avoid COVID-19 infection. COVID-19 may affect the processes and procedures for screening, diagnosis, and treatment of liver cirrhosis. • Cirrhosis, or scarring of the liver, can be caused by many chronic liver diseases, including viral hepatitis, as well as excessive alcohol intake, obesity, diabetes, diseases of the bile ducts, and a variety of toxic, metabolic, or other inherited diseases. • Most people with liver disease are asymptomatic. Complications, such as yellowing of the skin and eyes from jaundice, internal bleeding (varices), mental confusion (hepatic encephalopathy), and/or swollen belly from ascites, may take years to develop, so patients are often unaware of the severity of their condition and the slow, progressive damage.