Supplement 1A. Antineoplastic Medications Samples of the Intervention Group
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
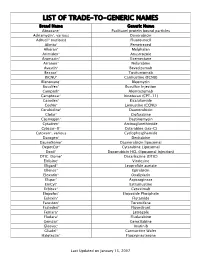
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

Table of Contents
ANTICANCER RESEARCH International Journal of Cancer Research and Treatment ISSN: 0250-7005 Volume 32, Number 4, April 2012 Contents Experimental Studies * Review: Multiple Associations Between a Broad Spectrum of Autoimmune Diseases, Chronic Inflammatory Diseases and Cancer. A.L. FRANKS, J.E. SLANSKY (Aurora, CO, USA)............................................ 1119 Varicella Zoster Virus Infection of Malignant Glioma Cell Cultures: A New Candidate for Oncolytic Virotherapy? H. LESKE, R. HAASE, F. RESTLE, C. SCHICHOR, V. ALBRECHT, M.G. VIZOSO PINTO, J.C. TONN, A. BAIKER, N. THON (Munich; Oberschleissheim, Germany; Zurich, Switzerland) .................................... 1137 Correlation between Adenovirus-neutralizing Antibody Titer and Adenovirus Vector-mediated Transduction Efficiency Following Intratumoral Injection. K. TOMITA, F. SAKURAI, M. TACHIBANA, H. MIZUGUCHI (Osaka, Japan) .......................................................................................................... 1145 Reduction of Tumorigenicity by Placental Extracts. A.M. MARLEAU, G. MCDONALD, J. KOROPATNICK, C.-S. CHEN, D. KOOS (Huntington Beach; Santa Barbara; Loma Linda; San Diego, CA, USA; London, ON, Canada) ...................................................................................................................................... 1153 Stem Cell Markers as Predictors of Oral Cancer Invasion. A. SIU, C. LEE, D. DANG, C. LEE, D.M. RAMOS (San Francisco, CA, USA) ................................................................................................ -

Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Acla
[CANCER RESEARCH 49. 4357-4362, August 1. 1989] Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Aclarubicin Hydrochloride 1omonimi Ichihara,1 Kiyoshi Sakamoto, Katsutaka Mori, and Masanobu Akagi Department of Surgery II, Kumamoto University Medical School, Kumamoto 860, Japan 4BSTRACT MATERIALS AND METHODS Transcatheter arterial Chemoembolization therapy using polylactic Preparation of PLA-ACRms acid microspheres containing aclarubicin hydrochloride (ACR) was per PLA-ACRms were prepared in the pharmacy of the Kumamoto formed in 62 patients with primary hepatocellular carcinoma. These microspheres were about 200 ¡anin diameter and contained 10% (w/w) University Hospital. Briefly, aclarubicin hydrochloride and isopropyl aclarubicin. A single dose of polylactic acid microspheres containing ACR myristate, a medium-chain fatty acid ester, were dissolved in 7.5% (50-100 mg of ACR) was administered 1 to 8 times with a mean of 2.2 polylactic acid-methylene chloride. The resultant solution was dispersed in 1% gelatin solution and sterilized in an autoclave for 20 min at doses (a total of 160 treatments) in 62 patients. Antitumor effects were 120°C;thismixture was then stirred with a magnetic stirrer at 500 rpm observed from the decrease in serum a-fetoprotein levels (82.1% of the patients) and in two dimensional size of tumor on computed tomography for 1 h. The resultant microspheres were collected by filtration through (93.6%). The cumulative survival rate was 54.3% at 1 year, 24.6% at 2 a membrane filter (3 //m pore diameter), rinsed 2 to 3 times (in 1 liter years, and 19.2% at 3 years, respectively, among 59 patients with of distilled water), and dried under reduced pressure for 5 days. -

A Phase II Study of Paclitaxel and Capecitabine As a First-Line Combination Chemotherapy for Advanced Gastric Cancer
British Journal of Cancer (2008) 98, 316 – 322 & 2008 Cancer Research UK All rights reserved 0007 – 0920/08 $30.00 www.bjcancer.com A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer Clinical Studies HJ Kang1, HM Chang1, TW Kim1, M-H Ryu1, H-J Sohn1, JH Yook2,STOh2, BS Kim2, J-S Lee1 and Y-K Kang*,1 1 2 Division of Oncology, Department of Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea; Department of Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea Paclitaxel and capecitabine, which have distinct mechanisms of action and toxicity profiles, have each shown high activity as single agents in gastric cancer. Synergistic interaction between these two drugs was suggested by taxane-induced upregulation of thymidine phosphorylase. We, therefore, evaluated the antitumour activity and toxicities of paclitaxel and capecitabine as first-line therapy in patients with advanced gastric cancer (AGC). Patients with histologically confirmed unresectable or metastatic AGC were treated À2 À2 with capecitabine 825 mg m p.o. twice daily on days 1–14 and paclitaxel 175 mg m i.v. on day 1 every 3 weeks until disease progression or unacceptable toxicities. Between June 2002 and May 2004, 45 patients, of median age 57 years (range ¼ 38–73 years), were treated with the combination of capecitabine and paclitaxel. After a median 6 cycles (range ¼ 1–9 cycles) of chemotherapy, 43 were evaluable for toxicity and response. A total of 2 patients showed complete response and 20 showed partial response making the overall response rate 48.9% (95% CI ¼ 30.3–63.5%). -

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Encapsulation of Nedaplatin in Novel Pegylated Liposomes Increases Its Cytotoxicity and Genotoxicity Against A549 and U2OS Human Cancer Cells
pharmaceutics Article Encapsulation of Nedaplatin in Novel PEGylated Liposomes Increases Its Cytotoxicity and Genotoxicity against A549 and U2OS Human Cancer Cells 1, 2, 1 1 2, Salma El-Shafie y, Sherif Ashraf Fahmy y , Laila Ziko , Nada Elzahed , Tamer Shoeib * and Andreas Kakarougkas 1,* 1 Department of Biology, School of Sciences and Engineering, The American University in Cairo, Cairo 11835, Egypt; [email protected] (S.E.-S.); [email protected] (L.Z.); [email protected] (N.E.) 2 Department of Chemistry, School of Sciences and Engineering, The American University in Cairo, Cairo 11835 Egypt; sheriff[email protected] * Correspondence: [email protected] (T.S.); [email protected] (A.K.) These authors contribute equally to this paper. y Received: 7 April 2020; Accepted: 25 August 2020; Published: 10 September 2020 Abstract: Following the discovery of cisplatin over 50 years ago, platinum-based drugs have been a widely used and effective form of cancer therapy, primarily causing cell death by inducing DNA damage and triggering apoptosis. However, the dose-limiting toxicity of these drugs has led to the development of second and third generation platinum-based drugs that maintain the cytotoxicity of cisplatin but have a more acceptable side-effect profile. In addition to the creation of new analogs, tumor delivery systems such as liposome encapsulated platinum drugs have been developed and are currently in clinical trials. In this study, we have created the first PEGylated liposomal form of nedaplatin using thin film hydration. Nedaplatin, the main focus of this study, has been exclusively used in Japan for the treatment of non-small cell lung cancer, head and neck, esophageal, bladder, ovarian and cervical cancer. -
Chapter 1.Pdf
CHAPTER 1 INTRODUCTION AND OUTLINE OF THIS THESIS INTRODUCTION Inflammatory bowel diseases (IBD), comprising Crohn’s disease, ulcerative colitis and IBD- 1 unclassified, are chronic recurrent inflammatory disorders of the (large) intestine with a heterogeneous, but often disabling disease presentation. The prevalence of IBD is increasing worldwide and medical therapies are introduced earlier to improve health-related quality of life and postpone surgical interventions1,2. This has led to the more extensive use of immunosuppressive therapies including biologicals and thiopurines. While the use of tioguanine was soon abandoned due to toxicity concerns3, over the years efficacy data on the use of azathioprine and mercaptopurine for the treatment of IBD has grown4,5. However, due to side effects and ineffectiveness, related to unprofitable metabolism, thiopurine therapy often fails; up to 50% of patients discontinues treatment within 2 years6. Increasing the knowledge on thiopurine metabolism may provide opportunities to further explore the therapeutic usefulness of thiopurines, which both from a patient’s perspective and a pharmacoeconomic perspective is essential. THIOPURINES In the 1950’s Gertrude Elion (1918-1999) worked as an assistant to George Hitchings (1905- 1998), both employees of Burroughs-Wellcome, focussing on the advent of drugs that inhibited cell growth without doing harm to the host cells. They discovered that purine bases are essential components of nucleic acids and vital for cell growth7. Subsequently, they designed the purine antagonists diaminopurine and tioguanine that blocked the synthesis of original nucleic acids and inhibited cell growth8. For the first time a successful treatment of leukaemia became available. Some years later Elion and colleagues also developed mercaptopurine, azathioprine, allopurinol, trimethoprim and acyclovir. -

WO 2017/176265 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date W O 2017/176265 A l 12 October 2017 (12.10.2017) P O P C T (51) International Patent Classification: (74) Agent: COLLINS, Daniel W.; Foley & Lardner LLP, A61K 9/00 (2006.01) A61K 9/51 (2006.01) 3000 K Street, NW, 6th Floor, Washington, DC 20007- A61K 47/42 (20 ) 5109 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/US20 16/026270 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) International Filing Date: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 6 April 2016 (06.04.2016) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, EST, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (26) Publication Language: English MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (71) Applicant: MAYO FOUNDATION FOR MEDICAL PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, EDUCATION AND RESEARCH [US/US]; 200 First SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, Street, NW, Rochester, Minnesota 55905 (US). TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (84) Designated States (72) Inventors: MARKOVIC, Svetomir N.; c/o Mayo Founda (unless otherwise indicated, for every tion For Medical Education And Research, 200 First kind of regional protection available): ARIPO (BW, GH, Street, NW, Rochester, Minnesota 55905 (US). -

Towards the Reduction of Occupational Exposure To
TOWARDS THE REDUCTION OF OCCUPATIONAL EXPOSURE TO CYTOTOXIC DRUGS by Cristian Vasile Barzan B.Sc. Simon Fraser University, 2007 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE in The Faculty of Graduate Studies (Occupational and Environmental Hygiene) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2010 © Cristian Vasile Barzan, 2010 Abstract Background : One of the most powerful and widely used techniques in cancer treatment is the use of cytotoxic drugs in chemotherapy. These drugs are inherently hazardous with many of them causing carcinogenic, mutagenic or teratogenic health outcomes. Occupational exposure to cytotoxic drugs is of great concern due to their lack of selectivity between healthy and unhealthy cells. Widespread cytotoxic drug contamination has been reported in North America, Europe and Australia. Current cleaning protocols for hazardous antineoplastic drugs include the use of disinfectants and oxidizing agents, such as household bleach. Aim : The thesis project focused on two objectives: 1) hypothesize and confirm potential hazardous by- products arising from cleaning cyclophosphamide, a widely used cytotoxic drugs, with household bleach, a commonly used cleaning agent; 2) develop an effective and safe cleaning agent for cytotoxic drugs in order to prevent and eliminate exposure to these drugs. Methods : The gas chromatograph mass spectrum (GC/MS) was used to analyze the decomposition of cyclophosphamide by household bleach (5.25% hypochlorite). The reaction was conducted in a test-tube and the by-products extracted and derivatized prior to analysis. Multiple cleaning agent compositions were tested on 10x10cm stainless steel plates spiked with the two model cytotoxic drugs, cyclophosphamide and methotrexate. -

Relevance Network Between Chemosensitivity and Transcriptome in Human Hepatoma Cells1
Vol. 2, 199–205, February 2003 Molecular Cancer Therapeutics 199 Relevance Network between Chemosensitivity and Transcriptome in Human Hepatoma Cells1 Masaru Moriyama,2 Yujin Hoshida, topoisomerase II  expression, whereas it negatively Motoyuki Otsuka, ShinIchiro Nishimura, Naoya Kato, correlated with expression of carboxypeptidases A3 Tadashi Goto, Hiroyoshi Taniguchi, and Z. Response to nimustine was associated with Yasushi Shiratori, Naohiko Seki, and Masao Omata expression of superoxide dismutase 2. Department of Gastroenterology, Graduate School of Medicine, Relevance networks identified several negative University of Tokyo, Tokyo 113-8655 [M. M., Y. H., M. O., N. K., T. G., H. T., Y. S., M. O.]; Cellular Informatics Team, Computational Biology correlations between gene expression and resistance, Research Center, Tokyo 135-0064 [S. N.]; and Department of which were missed by hierarchical clustering. Our Functional Genomics, Graduate School of Medicine, Chiba University, results suggested the necessity of systematically Chiba 260-8670 [N. S.], Japan evaluating the transporting systems that may play a major role in resistance in hepatoma. This may provide Abstract useful information to modify anticancer drug action in Generally, hepatoma is not a chemosensitive tumor, hepatoma. and the mechanism of resistance to anticancer drugs is not fully elucidated. We aimed to comprehensively Introduction evaluate the relationship between chemosensitivity and Hepatoma is a major cause of death even in developed gene expression profile in human hepatoma cells, by countries, and its incidence is increasing (1). Despite the using microarray analysis, and analyze the data by progress of therapeutic technique (2), the efficacy of radical constructing relevance networks. therapy is hampered by frequent recurrence and advance of In eight hepatoma cell lines (HLE, HLF, Huh7, Hep3B, the tumor (3). -

Title Second-Line Chemotherapy for Small-Cell Lung Cancer
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository Second-line chemotherapy for small-Cell Lung Cancer Title (SCLC). Author(s) Kim, Young Hak; Mishima, Michiaki Citation Cancer treatment reviews (2011), 37(2): 143-150 Issue Date 2011-04 URL http://hdl.handle.net/2433/137220 Right © 2010 Elsevier Ltd Type Journal Article Textversion author Kyoto University Second-line Chemotherapy for Small-Cell Lung Cancer (SCLC) Young Hak Kim and Michiaki Mishima Department of Respiratory Medicine, Graduate School of Medicine, Kyoto University, 54 Shogoin-Kawaharacho, Sakyo-ku, Kyoto 606-8507, Japan For reprints and all correspondence: Young Hak Kim Department of Respiratory Medicine, Graduate School of Medicine, Kyoto University, 54 Shogoin-Kawaharacho, Sakyo-ku, Kyoto 606-8507, Japan Phone: +81-75-751-3830; Fax: +81-75-751-4643; E-mail: [email protected] Running title: Second-line Chemotherapy for SCLC Key words: small-cell lung cancer, relapsed, chemotherapy, second line, sensitive, refractory 1 Abstract Although small-cell lung cancer (SCLC) generally shows an excellent response to initial chemotherapy, most patients finally relapse and salvage chemotherapy is considered. Usually, the response to salvage chemotherapy significantly differs between sensitive and refractory relapse. Sensitive relapse is relatively chemosensitive and re-challenge with the same drugs as used in the initial chemotherapy has been used historically, while refractory relapse is extremely chemoresistant and its prognosis has been abysmal. To date, a number of clinical trials have been carried out for relapsed SCLC; however, the number of randomized trials is quite limited.