57Thannual REPORT 2017-18
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Current Affairs of January 2020 Quick Point
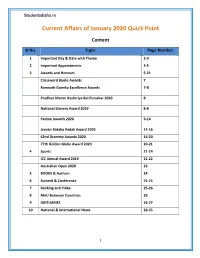
Studentsdisha.in Current Affairs of January 2020 Quick Point Content SI No. Topic Page Number 1 Important Day & Date with Theme 2-3 2 Important Appointments 3-5 3 Awards and Honours 5-21 Crossword Books Awards 7 Ramnath Goenka Excellence Awards 7-8 Pradhan Mantri Rashtriya Bal Puraskar 2020 8 National Bravery Award 2019 8-9 Padma Awards 2020 9-14 Jeevan Raksha Padak Award 2020 14-16 62nd Grammy Awards 2020 16-20 77th Golden Globe Award 2020 20-21 4 Sports 21-24 ICC Annual Award 2019 21-22 Australian Open 2020 22 5 BOOKS & Authors 24 6 Summit & Conference 24-25 7 Ranking and Index 25-26 8 MoU Between Countries 26 9 OBITUARIES 26-27 10 National & International News 28-35 1 Studentsdisha.in January 2020 Quick Point Important Day & Date with Theme of January 2020 Day Observation/Theme 1st Jan Global Family Day World Peace Day 4th Jan World Braille Day 6th Jan Journalists’ Day in Maharashtra 6th Jan The World Day of War Orphans 7th Jan Infant Protection Day 8th Jan African National Congress Foundation Day 9th Jan Pravasi Bharatiya Divas/NRI Day( 16th edition) 10thJan “World Hindi Day” 10thJan World Laughter Day 12th Jan National Youth Day or Yuva Diwas. Theme:"Channelizing Youth Power for Nation Building". 14th Jan Indian Armed Forces Veterans Day 15thJan Indian Army Day(72nd) 16thJan Religious Freedom day 18th Jan 15th Raising Day of NDRF(National Disaster Response Force) 19th Jan National Immunization Day (NID) 21st Jan Tripura, Manipur &Meghalaya 48th statehood day 23rdJan Subhash Chandra Bose Jayanti 24th to 30th National Girl Child Week Jan 24thJan National Girl Child Day Theme:‘Empowering Girls for a Brighter Tomorrow’. -

VETRII IAS STUDY CIRCLE TNPSC Current Affairs JANUARY - 2020
VETRII IAS STUDY CIRCLE TNPSC CURRENT AFFAIRS JANUARY - 2020 An ISO 9001 : 2015 Institution | Providing Excellence Since 2011 Head Office Old No.52, New No.1, 9th Street, F Block, 1st Avenue Main Road, (Near Istha siddhi Vinayakar Temple), Anna Nagar East – 600102. Phone: 044-2626 5326 | 98844 72636 | 98844 21666 | 98844 32666 Branches SALEM KOVAI No.189/1, Meyanoor Road, Near ARRS Multiplex, (Near Salem New No.347, D.S.Complex (3rd floor), Nehru Street,Near Gandhipuram bus Stand), Opp. Venkateshwara Complex, Salem - 636004. Central Bus Stand, Ramnagar, Kovai - 9 0427-2330307 | 95001 22022 75021 65390 Educarreerr Location Vivekanandha Educational Institutions for Women, Elayampalayam, Tiruchengode - TK Namakkal District - 637 205. 04288 - 234670 | 91 94437 34670 Patrician College of Arts and Science, 3, Canal Bank Rd, Gandhi Nagar, Opposite to Kotturpuram Railway Station, Adyar, Chennai - 600020. 044 - 24401362 | 044 - 24426913 Sree Saraswathi Thyagaraja College Palani Road, Thippampatti, Pollachi - 642 107 73737 66550 | 94432 66008 | 90951 66009 www.vetriias.com My Dear Aspirants, Greetings to all of you! “What we think we become” Gautama Buddha. We all have dreams. To make dreams come into reality it takes a lot of determination, dedication, self discipline and continuous effort. We at VETRII IAS Study Circle are committed to provide the right guidance, quality coaching and help every aspirants to achieve his or her life’s cherished goal of becoming a civil servant. The class room coaching at VETRII IAS Study Circle is meticulously planned to equip the aspirants with all the relevant facts and fundamentals of the subjects. Further the VETRII IAS Study Circle Study materials aim to support the candidate by providing the most relevant study material in a comprehensive manner. -

Pratibha Pages
17 Model Questions 1. Lt Gen Chandi Prasad Mohanty India got which position in the was appointed as the 1) Army chief 2) Vice Chief of Army Staff 3) Navy Chief 4) Airforce chief 5) Vice chief of navy staff Democracy Index - 2020? 2. Which tableau was conferred with the Best tableau award during n e-mail: [email protected] ñªëÅ]î¦ô¢Ù íƇvñ÷J 10, 2021 Republic Day parade? 1) Punjab themed '400th Birth National with an outlay of Rs 2.87 lakh crore show had seen several aircraft of the Anniversary of Sri Guru Tegh over 5 years which is aimed at a uni- Indian Air Force (IAF), Army, Navy, Bahadur' Union BUDGET 2021-22 versal water supply in all 4,378 urban Hindustan Aeronautics (HAL), and the General 2) Uttarkhand themed 'DevBhoomi' local bodies. Coast Guard. On 1 February 2021, the finance minis- 3) Uttar Pradesh themed 'Ayodhya: The minister said that in FY22 outlay While the indigenously developed The cultural Heritage of Uttar ter Nirmala Sitharaman presented her (budget estimate) for health & well- Tejas-LCA helicopters and Surya Kiran Awareness Pradesh' first paperless union budget in the par- being is up 138%, and is at Rs 2,23,846 aircraft stole the show, Sukhoi, Rafale, 4) West Bengal themed 'Sabooj liament. She mentioned that this year’s crore. Govt has also allocated Hawk and the American B-1B Lancer Head Constable Shyam Narayan Sathi- Wheels of Change' budget proposals rest on six pillars - Rs 35,400 crore towards COVID vac- heavy bomber were among the star Singh Yadav, CRPF Constable Vinod physical, financial capital and infra- cines for FY22 and is committed to attractions. -

The Political Economy of Hindu Nationalism in India 1998-2004
THE POLITICAL ECONOMY OF HINDU NATIONALISM IN INDIA 1998-2004 submitted for the degree of Doctor of Philosophy Politics and International Relations John Joseph Abraham Royal Holloway, University of London 1 2 Declaration of Authorship I John Joseph Abraham hereby declare that this thesis and the work presented in it is entirely my own. Where I have consulted the work of others, this is always clearly stated. Signed: John Joseph Abraham August 22, 2014 3 4 Acknowledgements I would like to express my gratitude to a number of people who have made this project possible. I thank my supervisors Dr. Yasmin Khan and Dr. Oliver Heath for their careful guidance, constant support and enthusiasm over these years. Thanks is also due to Dr. James Sloam for his insights at important stages of this project. Finally I would like to thank Dr. Tony Charles for his valuable support in the final stages of this work. I thank Dr. Nathan Widder under whose leadership the Department of Politics and International Relations has been a supportive environment and congenial forum for the development of ideas and Dr. Jay Mistry, Dr. Ben O'Loughlin, Dr. Sandra Halperin and Anne Uttley for the important roles they have played in my development as an academic scholar. Finally, thanks is due to my fellow researchers, Shyamal Kataria, Baris Gulmez, Didem Buhari, Celine Tschirhart, Ali Mosadegh Raad, Braham Prakash Guddu and Mark Pope for the many useful conversations and sympathetic understanding. This project would have not been possible but for the help of my family. I would like to thank my parents Abraham and Valsa Joseph as well as George and Annie Mathew for their constant encouragement and eager support. -

Title Title Daily Current Affairs Capsule 27Th January 2021
Title Daily Current Affairs Capsule th Title 27 January 2021 International Day of Commemoration in memory of the victims of the Holocaust: 27 January International Day of Commemoration in memory of the victims of the Holocaust (International Holocaust Remembrance Day) is observed on 27 January every year. The day commemorates the anniversary of the tragedy of the Holocaust that occurred during the Second World War. A genocide occurred during World War II in which Nazi Germany, aided by its collaborators, systematically murdered some six million European Jews, around two-thirds of the Jewish population of Europe, between 1941 and 1945. The theme of the International Holocaust Remembrance Day 2021 is “Facing the Aftermath: Recovery and Reconstitution after the Holocaust” Italian PM Conte resigns Italian Prime Minister Giuseppe Conte resigned after losing his Senate majority, plunging the country into political uncertainty just as it’s battling the pandemic and a recession. He tendered his resignation to President Sergio Mattarella, the ultimate arbiter of Italian political crises, who invited him to stay on in a caretaker capacity pending discussions on what happens next. Italy was the first European country to face the full force of the Covid-19 pandemic and has since suffered badly, with the economy plunged into recession and deaths still rising by around 400 a day. Parts of the country remain under partial lockdown, the vaccination programme has slowed and a deadline is looming to agree plans to spend billions of euros in European Union recovery funds. Tamil Nadu CM inaugurates Jayalalithaa Memorial in Chennai Tamil Nadu Chief Minister Edappadi Palanisamy inaugurated Jayalalithaa Memorial at Chennai. -

Download Brochure
Celebrating UNESCO Chair for 17 Human Rights, Democracy, Peace & Tolerance Years of Academic Excellence World Peace Centre (Alandi) Pune, India India's First School to Create Future Polical Leaders ELECTORAL Politics to FUNCTIONAL Politics We Make Common Man, Panchayat to Parliament 'a Leader' ! Political Leadership begins here... -Rahul V. Karad Your Pathway to a Great Career in Politics ! Two-Year MASTER'S PROGRAM IN POLITICAL LEADERSHIP AND GOVERNMENT MPG Batch-17 (2021-23) UGC Approved Under The Aegis of mitsog.org I mitwpu.edu.in Seed Thought MIT School of Government (MIT-SOG) is dedicated to impart leadership training to the youth of India, desirous of making a CONTENTS career in politics and government. The School has the clear § Message by President, MIT World Peace University . 2 objective of creating a pool of ethical, spirited, committed and § Message by Principal Advisor and Chairman, Academic Advisory Board . 3 trained political leadership for the country by taking the § A Humble Tribute to 1st Chairman & Mentor, MIT-SOG . 4 aspirants through a program designed methodically. This § Message by Initiator . 5 exposes them to various governmental, political, social and § Messages by Vice-Chancellor and Advisor, MIT-WPU . 6 democratic processes, and infuses in them a sense of national § Messages by Academic Advisor and Associate Director, MIT-SOG . 7 pride, democratic values and leadership qualities. § Members of Academic Advisory Board MIT-SOG . 8 § Political Opportunities for Youth (Political Leadership diagram). 9 Rahul V. Karad § About MIT World Peace University . 10 Initiator, MIT-SOG § About MIT School of Government. 11 § Ladder of Leadership in Democracy . 13 § Why MIT School of Government. -

Draft Report of the PAC on 2G and 3G Spectrum Allocation
REPORT PART – I CHAPTER - I BACKGROUND 1.1 In recent times, India has emerged as one of the most dynamic and promising and fastest growing telecom markets in the world. It has third largest overall telecom network and the second largest wireless network in the world. Mobile telephony and thus Spectrum have played a vital role in the stupendous growth of the telecom services in India. The word ‘Spectrum’ basically refers to a collection of various types of electromagnetic radiations of different wavelengths. Radio frequency Spectrum is a limited global natural resource with a high economic value, due to its heavy demand in the telecommunication sector. It is a finite but non-consumable natural resource. But it will be wasted if not used efficiently. In India, the radio frequencies are being used for around forty different types of services like space communication, mobile communication, broadcasting, radio navigation, mobile satellite service, aeronautical satellite services, defence communication etc. 1.2 Some of the important and typical characteristics of the radio frequency Spectrum are as below: (i) Radio frequency spectrum does not respect international geographical boundaries as it is spread over a large terrestrial area. (ii) Use of radio frequency spectrum is susceptible to overlapping interference and requires the application of complex engineering tools to ensure interference free operation of various wireless networks. (iii) Unlike other natural resources, radio frequency spectrum is not consumed upon its usage. It is also liable to be wasted if it is not used optimally and efficiently. Radio frequency spectrum usage is, therefore, to be shared amongst the various radio services and must be used efficiently, optimally and economically in conformity with the provisions of national and international laws. -

Daily Current Affairs for App 20201213
Daily Current Affairs for app_20201213 Biden signs 'Buy American' order, vows to strengthen manufacturing sector President Joe Biden vowed to leverage the purchasing power of the U.S. government, the world's biggest single buyer of goods and services, to strengthen domestic manufacturing and create markets for new technologies. The Democratic president signed an executive order aimed at closing loopholes in existing "Buy American" provisions, which apply to about a third of the $600 billion in goods and services the federal government buys each year. The order will make any waivers more transparent and create a senior White House role to oversee the process. China overtook the United States as the world's top manufacturer in 2010, and was responsible for 28% of global output in 2018, according to United Nations data. 50 lakh doses of COVID 19 vaccine from Serum Institute of India arrive in Dhaka The first instalment of 50 lakh doses of the COVID 19 vaccine from Serum Institute of India (SII) arrived in Dhaka. This is part of the agreement for the purchase of 30 million doses of the vaccine for which Beximco Pharmaceuticals Limited (BPL) had signed a tripartite agreement with the SII on December 13 last year. Prime Minister Sheikh Hasina is scheduled to inaugurate the vaccination programme in Dhaka from Wednesday. A nurse of the Kurmitola general hospital in Dhaka will receive the first vaccine shot. Daily Current Affairs for app_20201213 Liberation War fighter Lt. Col Quazi Sajjad Ali Zahir and Musicologist Sanjida Khatun from Bangladesh awarded Padma Shree Bangladesh Liberation War fighter Lt. -

The Making of Regulatory Independence By
THE MAKING OF REGULATORY INDEPENDENCE BY SIDDHARTHA SUNDER RAJA DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Communication in the Graduate College of the University of Illinois at Urbana-Champaign, 2011 Urbana, Illinois Doctoral Committee: Associate Professor Christian Sandvig, Chair Professor Peggy Miller Professor Dan Schiller Professor Noshir Contractor Abstract This study offers an ethnographic account of life at a regulatory agency to offer a new perspective on an important question: how does a regulatory agency become and remain independent? Relying on an analytical framework based on scholarship in legal anthropology, this study provides elements of an answer based on an insider‘s view of regulation, illuminating the complex, messy, and political nature of what may seem from the outside as calm and neutral application of technical expertise. The formal account suggests that legislative action defines the position and mandate of such an agency making it independent—immune from political influence in its decision-making. However, experience has shown that the making and maintenance of independence is a challenge, especially as these agencies typically enter arenas much after other powerful economic and political interests have established their own positions. The result is that in spite of efforts of international financial institutions, governments, and regulatory staffers worldwide to create independent regulatory agencies, many of these agencies are severely constrained in their ability to function effectively. Using unprecedented access to individuals working at the Telecom Regulatory Authority of India (TRAI), this study will show that regulatory staffers are constantly struggling to make and maintain their position, define their role, and keep their agency going. -

Vol.13 Jan-Mar 2012
Covering developments on policy responses, policy implementation and policy distortions on a quarterly basis. Comments are welcome. VolumePP 13,oo No. 1 licyWatchlicyWatch January-March 2012 Catch the Signal of Change s India a banana republic or a dirigisme economy? Neither. Firstly, we Iare not a small country with a single export item, and we are neither a state-controlled economy. However, there is a preponderance of both elements in our governance system. In a colloquial sense, we are no better than a banana republic, where public interest is given the short shrift and crony I N S I D E T H I S I S S U E capitalism rules. The state does control a large TRAI to set up part of our economy, and has Complaint Centre .................3 failed in allocating them in a fair Panel to Resolve manner to the best possible bidder, whether it is minerals or oil or Power Woes .........................6 www.bollyguide.com spectrum. And private interest 100% FDI in Single overrides public interest, coupled Brand Retail ....................... 10 with unjust enrichment of the Malnutrition a polity, babus and businesses. National Shame ............... 12 The doctrine of public interest Coal gate ......................... 14 has been reinforced again and again in all recent Supreme Court judgments. The latest one on cancellation of 122 mobile telephony 2G licences has been Vocational Education the most daring and forward-looking. This order will have an impact on Framework ........................ 18 other sectors as well as the whole governance system. The apex court is doing what the government should have done.The government has been behaving in a cavalier fashion, for obvious reasons. -

Download RRB Group D Polity Notes in English
www.gradeup.co 1 www.gradeup.co 1.Which one of the following is a violation of the right to life or personal liberty? A. The arrested person is informed about the reason of his arrest. B. He is produced before the court within 24 hours of his arrest. C. He is not allowed to consult his lawyer. D. He is shown the warrant before arrest. Ans. C Sol. If the person is not allowed to consult his lawyer that is a violation of the right to life or personal liberty. 2.The words 'Satyameva Jayate' have been taken from A. Vedas B. Bhagwad Gita C. Mundaka Upanishada D. Mahabharata Ans. C Sol. Satyameva Jayate is a mantra from the ancient Indian scripture Mundaka Upanishad. Following the independence of India, it was adopted as the national motto of India in 26 January 1950. It is inscribed in script at the base of the national emblem. 3.The Chief Minister of Goa as of March 2018, Manohar Panikar, belongs to: A. Janata Dal Party B. Indian National Congress C. Bharatiya Janata Party D. CPI Ans. C Sol. ● The Chief Minister of Goa as of March 2018, Manohar Panikar, belongs to Bharatiya Janata Party. ● He was the 10th chief minister of Goa. ● He was posthumously awarded Padma Bhushan in 2020. ● The Indian Institute for Defense Studies and Analyses was renamed the Manohar Parrikar Institute for Defense Studies and Analyses. 4.As of August 2018, what is the portfolio held by the current Union Minister Smriti Irani? A. Information and Broadcasting B. Science and Technology C. -

National Affairs
NATIONAL AFFAIRS Prithvi II Missile Successfully Testifi ed India on November 19, 2006 successfully test-fi red the nuclear-capsule airforce version of the surface-to- surface Prithvi II missile from a defence base in Orissa. It is designed for battlefi eld use agaisnt troops or armoured formations. India-China Relations China’s President Hu Jintao arrived in India on November 20, 2006 on a fourday visit that was aimed at consolidating trade and bilateral cooperation as well as ending years of mistrust between the two Asian giants. Hu, the fi rst Chinese head of state to visit India in more than a decade, was received at the airport in New Delhi by India’s Foreign Minister Pranab Mukherjee and Science and Technology Minister Kapil Sibal. The Chinese leader held talks with Indian Prime Minister Manmohan Singh in Delhi on a range of bilateral issues, including commercial and economic cooperation. The two also reviewed progress in resolving the protracted border dispute between the two countries. After the summit, India and China signed various pacts in areas such as trade, economics, health and education and added “more substance” to their strategic partnership in the context of the evolving global order. India and China signed as many as 13 bilateral agreements in the presence of visiting Chinese President Hu Jintao and Prime Minister Manmohan Singh. The fi rst three were signed by External Affairs Minister Pranab Mukherjee and Chinese Foreign Minister Li Zhaoxing. They are: (1) Protocol on the establishment of Consulates-General at Guangzhou and Kolkata. It provides for an Indian Consulate- General in Guangzhou with its consular district covering seven Chinese provinces of Guangdong, Fujian, Hunan, Hainan, Yunnan, Sichuan and Guangxi Zhuang autonomous region.