Who Study Group on Tobacco Product Regulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Roger Lee Papke Box 100267 JHM Health Science Center Gainesville, Florida 32610
CURRICULUM VITAE Roger Lee Papke DEPARTMENT OF PHARMACOLOGY AND THERAPEUTICS UNIVERSITY OF FLORIDA SCHOOL OF MEDICINE Box 100267 J.H.M. Health Science Center Gainesville, Florida 32610 (352) 392-4712, FAX (352) 392-9696 [email protected] BIOGRAPHICAL DATA: Born: October 12, 1953, Kenmore, New York Married: December 24, 1980 to Clare Stokes Citizenship: U. S. A. EDUCATION: Starpoint Central School Pendleton, N. Y. Primary and Secondary N.Y.S. Regents Diploma 1971 New York University Washington Square College of Arts and Sciences 1971 - 1975 Majors in Biology and Classical Civilization Bachelor of Arts awarded May 1975 New York University Graduate school of Arts and Sciences 1975 - 1976 Thesis advisor: Dr. Fleur L. Strand Thesis title: An Alpha Adrenergic Response of Cardiac Muscle at an Alkaline pH Master of Science awarded May 1976 Cornell University Graduate School of Arts and Science 1976-1979: Section of Physiology Graduate Research Assistant in Reproductive Physiology Advisor: Dr. William Hansel Research topic: The endocrine control of delayed implantation in mink Cornell University Graduate School of Arts and Science 1979-1986: Section of Neurobiology and Behavior Thesis Advisor: Dr. Robert Oswald Primary research topic: Pharmacology of nicotinic acetylcholine receptors Thesis Title: The Gating of Single Channel Currents Through the Nicotinic Acetylcholine Receptors of BC3H-1 Cells: Effects of Agonists and Allosteric Ligands Ph.D. conferred January 1987 ACADEMIC APPOINTMENTS: 1987 Postdoctoral Research Associate: Department of Pharmacology, -

Differential Effects of Alkaloids on Memory in Rodents
www.nature.com/scientificreports OPEN Diferential efects of alkaloids on memory in rodents Patrick M. Callahan1,2, Alvin V. Terry Jr.1,2, Manuel C. Peitsch3, Julia Hoeng3* & Kyoko Koshibu3* Nicotinic acetylcholine receptors (nAChRs) play a critical role in the neuropharmacology of learning and memory. As such, naturally occurring alkaloids that regulate nAChR activity have gained interest for understanding and potentially improving memory function. In this study, we tested the acute efects of three known nicotinic alkaloids, nicotine, cotinine, and anatabine, in suppressing scopolamine-induced memory defcit in rodents by using two classic memory paradigms, Y-maze and novel object recognition (NOR) in mice and rats, respectively. We found that all compounds were able to suppress scopolamine-induced spatial memory defcit in the Y-maze spontaneous alternation paradigm. However, only nicotine was able to suppress the short-term object memory defcit in NOR, despite the higher doses of cotinine and anatabine used to account for their potential diferences in nAChR activity. These results indicate that cotinine and anatabine can uniquely regulate short-term spatial memory, while nicotine seems to have more robust and general role in memory regulation in rodents. Thus, nAChR-activating alkaloids may possess distinct procognitive properties in rodents, depending on the memory types examined. Te cholinergic system of the brain is a critical regulator of attention, memory, and higher-order cognitive processing, and its defcits are central to the etiology of dementia1. As such, nicotinic acetylcholine receptors (nAChRs) have gained much interest as a target of drug development 2. Neuronal nAChRs are pentameric pro- teins composed of various combinations of α (α2–α9) and β (β2–β4) nAChR subunits, diferentially expressed throughout the nervous system. -

2016 Questionnaire
N R P F S S Administrative Use Only Nebraska Risk & School Name: Protective Factor Student Survey School ID: School District: Year 2016 The purpose of this survey is to learn how students in our schools feel about their community, family, peers, and school. The survey also asks about health behaviors. - The survey is completely voluntary and anonymous. Do NOT put your name on the questionnaire. - This is not a test, so there are no right or wrong answers. We would like you to work quickly so you can finish. - All of the questions should be answered by completely filling in one of the answer spaces. If you do not find an answer that fits exactly, use the one that comes closest. If any question does not apply to you, or you are not sure what it means, just leave it blank. You can skip any question that you do not wish to answer. - Mark only one answer to each question unless instructed otherwise. About You Your Experiences at School 1 Are you: 6 Putting them together, what were your grades Male like last year ? (Mark the one best answer.) Female Mostly F's Mostly D's Mostly C's 2 How old are you? Mostly B's 12 or younger 16 Mostly A's 13 17 14 18 15 19 or older 7 How interesting are most of your courses to you? Very interesting and stimulating Quite interesting 3 What grade are you in? Fairly interesting 7th 10th Slightly dull 8th 11th Very dull 9th 12th 8 How important do you think the things you are learning in school are going to be for your later life? 4 Are you Hispanic or Latino? Yes (Hispanic or Latino) Very important Quite important -

3.1 Measuring Tobacco Use Behaviours
chap3.1janvier13:Layout 1 13/01/2009 09:55 Page 75 3.1 Measuring tobacco use behaviours Introduction semination of tobacco-related Natural history of tobacco use surveillance data.” The majority of tobacco control In addition, Section 1-d of Article The natural history of tobacco use is policies are designed to reduce 21 requires each ratifying nation to often conceptualized as a series of tobacco use or exposure to tobacco provide periodic updates on sur- steps that can progress from never smoke in the environment; stra- veillance and research as specified use, to trial, experimentation, estab- tegies that are clearly supported by in Article 20. Article 22 calls for lished use, attempting to quit, the scientific literature (US cooperation among the Parties to relapse, and/or maintenance of Department of Health and Human promote the transfer of technical cessation (Figure 3.1 and Table 3.1) Services, 2004, 2006; IARC, 2004, and scientific expertise on sur- (US Department of Health and 2007a). Preventing initiation and veillance and evaluation, among Human Services, 1990, 1994; promoting quitting are the two major other topics (WHO, 2003). Marcus et al. , 1993; Pierce et al ., tobacco control strategies designed This section will first review the 1998b; Mayhew et al., 2000; Choi et to reduce use. To facilitate pro- natural history of tobacco use (e.g. al. , 2001; Hughes et al ., 2003). Prior gress, article 20 of the WHO initiation, current use, cessation). In to actual initiation of use, never Framework Convention on Tobacco epidemiologic studies of disease users often think about use, a step Control (FCTC) calls for Parties to: etiology, such as those discussed in in the process that is described in IARC Monographs (e.g. -

Cincinnati Region
Ohio Substance Abuse Monitoring Network Drug Abuse Trends in the Cincinnati Region June 2010-January 2011 John R. Kasich, Governor Orman Hall, Director FAYETTE BUTLER WARREN CLINTON ROSS HAMILTON HIGHLAND PIKE CLERMONT BROWN ADAMS SCIOTO LAWRENCE Regional Epidemiologist: Jan Scaglione, BS, MT, PharmD, DABAT OSAM Staff: R. Thomas Sherba, PhD, MPH, LPCC Principal Investigator Rick Massatti, MSW Research Administrator • Ohio Department of Alcohol and Drug Addiction Services • Division of Planning, Outcomes & Research • 280 N. High St., 12th floor, Columbus, OH 43215• 1-800-788-7254 • www.odadas.ohio.gov • Cincinnati Region Surveillance of Drug Abuse Trends in the State of Ohio Cincinnati Region Regional Profile Indicator1 Ohio Cincinnati Region OSAM Drug Consumers Total Population, 2009 estimate 11,514,603 2,053,493 38 Gender (Female), 2009 51.2% 51.1% 50.0% Whites, 2009 82.2% 83.2% 47.4% African Americans, 2009 11.9% 12.6% 47.4% Hispanic or Latino Origin, 2009 2.8% 2.0% 2.6% High school graduates, 2008 84.6% 89.9% 76.3% Median household income, 2009 $45,467 $41,672 Less than $12,000 Persons below poverty, 2009 15.1% 16.2% 48.6%2 Ohio and Cincinnati statistics are derived from the U.S. Census Bureau1. Respondents reported income by selecting a category that best represented their household’s approximate income for 20092. Poverty status was unable to be determined for three respondents due to missing or insufficient income data3. Drug Consumer Characteristics (N=38) Male 19 Female 19 20's 17 30's 9 40's 6 50's + 6 Less than high school graduate -
FY 2020 Announcement.Pdf
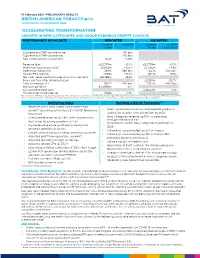
17 February 2021 –PRELIMINARY RESULTS BRITISH AMERICAN TOBACCO p.l.c. YEAR ENDED 31 DECEMBER 2020 ‘ACCELERATING TRANSFORMATION’ GROWTH IN NEW CATEGORIES AND GROUP EARNINGS DESPITE COVID-19 PERFORMANCE HIGHLIGHTS REPORTED ADJUSTED Current Vs 2019 Current Vs 2019 rates Rates (constant) Cigarette and THP volume share +30 bps Cigarette and THP value share +20 bps Non-Combustibles consumers1 13.5m +3.0m Revenue (£m) £25,776m -0.4% £25,776m +3.3% Profit from operations (£m) £9,962m +10.5% £11,365m +4.8% Operating margin (%) +38.6% +380 bps +44.1% +100 bps2 Diluted EPS (pence) 278.9p +12.0% 331.7p +5.5% Net cash generated from operating activities (£m) £9,786m +8.8% Free cash flow after dividends (£m) £2,550m +32.7% Cash conversion (%)2 98.2% -160 bps 103.0% +650 bps Borrowings3 (£m) £43,968m -3.1% Adjusted Net Debt (£m) £39,451m -5.3% Dividend per share (pence) 215.6p +2.5% The use of non-GAAP measures, including adjusting items and constant currencies, are further discussed on pages 48 to 53, with reconciliations from the most comparable IFRS measure provided. Note – 1. Internal estimate. 2. Movement in adjusted operating margin and operating cash conversion are provided at current rates. 3. Borrowings includes lease liabilities. Delivering today Building A Better TomorrowTM • Revenue, profit from operations and earnings • 1 growth* absorbing estimated 2.5% COVID-19 revenue 13.5m consumers of our non-combustible products , headwind adding 3m in 2020. On track to 50m by 2030 • New Categories revenue up 15%*, accelerating • Combustible revenue -
DISTRICT of COLUMBIA TOBACCO DIRECTORY Last Updated 08/15/19 Page 1 of 4
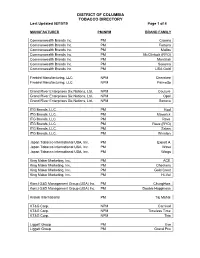
DISTRICT OF COLUMBIA TOBACCO DIRECTORY Last Updated 08/15/19 Page 1 of 4 MANUFACTURER PM/NPM BRAND FAMILY Commonwealth Brands Inc. PM Crowns Commonwealth Brands Inc. PM Fortuna Commonwealth Brands Inc. PM Malibu Commonwealth Brands Inc. PM McClintock (RYO) Commonwealth Brands Inc. PM Montclair Commonwealth Brands Inc. PM Sonoma Commonwealth Brands Inc. PM USA Gold Firebird Manufacturing, LLC NPM Cherokee Firebird Manufacturing, LLC NPM Palmetto Grand River Enterprises Six Nations, Ltd. NPM Couture Grand River Enterprises Six Nations, Ltd. NPM Opal Grand River Enterprises Six Nations, Ltd. NPM Seneca ITG Brands, LLC. PM Kool ITG Brands. LLC. PM Maverick ITG Brands, LLC. PM Rave ITG Brands, LLC. PM Rave (RYO) ITG Brands, LLC. PM Salem ITG Brands, LLC. PM Winston Japan Tobacco International USA, Inc. PM Export A Japan Tobacco International USA, Inc. PM Wave Japan Tobacco International USA, Inc. PM Wings King Maker Marketing, Inc. PM ACE King Maker Marketing, Inc. PM Checkers King Maker Marketing, Inc. PM Gold Crest King Maker Marketing, Inc. PM Hi-Val Konci G&D Management Group (USA) Inc. PM ChungHwa Konci G&D Management Group (USA) Inc. PM Double Happiness Kretek International PM Taj Mahal KT&G Corp. NPM Carnival KT&G Corp. NPM Timeless Time KT&G Corp. NPM This Liggett Group PM Eve Liggett Group PM Grand Prix DISTRICT OF COLUMBIA TOBACCO DIRECTORY Last Updated 08/15/19 Page 2 of 4 MANUFACTURER PM/NPM BRAND FAMILY Liggett Group PM Liggett Select Liggett Group PM Montego Liggett Group PM Pyramid NASCO PM Red Sun NASCO PM SF Ohserase Manufacturing LLC NPM Signal Peter Stokkebye Tobaksfabrik A/S PM Amsterdam Shag (RYO) Peter Stokkebye Tobaksfabrik A/S PM Danish Export (RYO) Peter Stokkebye Tobaksfabrik A/S PM London Export (RYO) Peter Stokkebye Tobaksfabrik A/S PM Norwegian Shag (RYO) Peter Stokkebye Tobaksfabrik A/S PM Stockholm Blend (RYO) Peter Stokkebye Tobaksfabrik A/S PM Turkish Export (RYO) Philip Morris USA Inc. -

Current Status of the Reduced Propensity Ignition Cigarette Program in Hawaii
Hawaii State Fire Council Current Status of the Reduced Propensity Ignition Cigarette Program in Hawaii Submitted to The Twenty-Eighth State Legislature Regular Session June 2015 2014 Reduced Ignition Propensity Cigarette Report to the Hawaii State Legislature Table of Contents Executive Summary .…………………………………………………………………….... 4 Purpose ..………………………………………………………………………....................4 Mission of the State Fire Council………………………………………………………......4 Smoking-Material Fire Facts……………………………………………………….............5 Reduced Ignition Propensity Cigarettes (RIPC) Defined……………………………......6 RIPC Regulatory History…………………………………………………………………….7 RIPC Review for Hawaii…………………………………………………………………….9 RIPC Accomplishments in Hawaii (January 1 to June 30, 2014)……………………..10 RIPC Future Considerations……………………………………………………………....14 Conclusion………………………………………………………………………….............15 Bibliography…………………………………………………………………………………17 Appendices Appendix A: All Cigarette Fires (State of Hawaii) with Property and Contents Loss Related to Cigarettes 2003 to 2013………………………………………………………18 Appendix B: Building Fires Caused by Cigarettes (State of Hawaii) with Property and Contents Loss 2003 to 2013………………………………………………………………19 Appendix C: Cigarette Related Building Fires 2003 to 2013…………………………..20 Appendix D: Injuries/Fatalities Due To Cigarette Fire 2003 to 2013 ………………....21 Appendix E: HRS 132C……………………………………………………………...........22 Appendix F: Estimated RIPC Budget 2014-2016………………………………...........32 Appendix G: List of RIPC Brands Being Sold in Hawaii………………………………..33 2 2014 -

TOBACCO WORLD RETAIL PRICES (Ovor 5,000 Retail PI-ICM)
THE CIGAR AND THE TOBACCO WORLD THE POPULAR JOURNAL TOBACCO OVER 40 YEARS OF TRADE USEFULNESS WORLD The Subscription includes : TOBACCO WORLD RETAIL PRICES (Ovor 5,000 Retail PI-ICM). RETAIL PRICES THE TOBACCO WORLD ANNUAL (Containing a word of Trad* Brand*—with Nam* and Addrau In each cms*). Membership of: TOBACCO WORLD SERVICE JUNE 1935 (With Pott Fnta raplUa In all Trad* difficult!**). The Cigar & Tobacco World HIYWOOO A COMPANY LTD. Dmrr How*, Kin—U 3tr*M, Ontry Una, London, W.C1 trantfc OACM f Baadmur. •trmlnfhtn, Uteanar. ToWfTHM i OffUlfrunt, Phono, LonAon. •Phono I TomaU far M1J Published by THE CIGAR & TOBACCO WORLD HEYWOOD & CO., LTD. DRURY HOUSE, RUSSELL STREET, DRURY LANE, LONDON, W.C.2 Branch Offices: MANCHESTER, BIRMINGHAM, LEICESTER Talagrarm : "Organigram. Phono, London." Phono : Tampla Bar MZJ '' Inar) "TOBACCO WORLD" RETAIL PRICES 1935 Authorised retail prices of Tobaccos, Cigarettes, Fancy Goods, and Tobacconists' Sundries. ABDULLA & Co., Ltd. (\BDULD^ 173 New Bond Street, W.l. Telephone; Bishopsgnte 4815, Authorised Current Retail Prices. Turkish Cigarettes. Price per Box of 100 50 25 20 10 No. 5 14/6 7/4 3/8 — 1/6 No. 5 .. .. Rose Tipped .. 28/9 14/6 7/3 — 3/- No. 11 11/8 5/11 3/- - 1/3 No. II .. .. Gold Tipped .. 13/S 6/9 3/5 - No. 21 10/8 5/5 2/9 — 1/1 Turkish Coronet No. 1 7/6 3/9 1/10J 1/6 9d. No. "X" — 3/- 1/6 — — '.i^Sr*** •* "~)" "Salisbury" — 2/6 — 1/- 6d. Egyptian Cigarettes. No. 14 Special 12/5 6/3 3/2 — — No. -

BAT INTERNATIONAL FINANCE Plc
BASE PROSPECTUS B.A.T. INTERNATIONAL FINANCE p.l.c. (incorporated with limited liability in England and Wales) BRITISH AMERICAN TOBACCO HOLDINGS (THE NETHERLANDS) B.V. (incorporated with limited liability in The Netherlands) B.A.T. NETHERLANDS FINANCE B.V. (incorporated with limited liability in The Netherlands) B.A.T CAPITAL CORPORATION (incorporated with limited liability in the State of Delaware, United States of America) £25,000,000,000 Euro Medium Term Note Programme unconditionally and irrevocably guaranteed by BRITISH AMERICAN TOBACCO p.l.c. (incorporated with limited liability in England and Wales) and each of the Issuers (except where it is the relevant Issuer) On 6 July 1998, each of B.A.T. International Finance p.l.c. (“BATIF”), B.A.T Capital Corporation (“BATCAP”) and B.A.T Finance B.V. (“BATFIN”) entered into a Euro Medium Term Note Programme (the “Programme”) for the issue of Euro Medium Term Notes (the “Notes”). On 16 April 2003, British American Tobacco Holdings (The Netherlands) B.V. (“BATHTN”) acceded to the Programme as an issuer and, where relevant, a guarantor and BATFIN was removed as an issuer and a guarantor under the Programme. On 9 December 2011, BATCAP was removed as an issuer and a guarantor under the Programme. On 16 May 2014, B.A.T. Netherlands Finance B.V. (“BATNF”) acceded to the Programme as an issuer and, where relevant, a guarantor. On 31 May 2017, BATCAP acceded to the Programme as an issuer and, where relevant, a guarantor. BATIF, BATHTN, BATNF and BATCAP are each, in their capacities as issuers under the Programme, an “Issuer” and together referred to as the “Issuers”. -

The Use of Pressure Drop Measurements for Estimating Ventilation and Paper Porosity* by C
Beitrage zur Tabakforschung International ·Volume 10 ·No. 1 ·December 1979 The Use of Pressure Drop Measurements for Estimating Ventilation and Paper Porosity* by C. H. Keith Celanese Fibers Company, Charlotte, North Carolina, U.S.A. The recent increase in the number and volume of ven 20 °C. For measurements on ventilated filters, the perti tilated cigarette brands has created a need for a simple, nent readings are the normal open cigarette pressure drop, non-destructive means of measuring the degree of air L\p 0 , the cigarette pressure drop with the filter vents dilution. Two techniques currently in use require the use closed, L\ p0, and the filter pressure drop, L\ Pf· For measure of auxiliary equipment or specialized analyses. One of ments on tobacco columns, a fully encapsulated pressure these is the sleeve method of Norman (1) which encloses drop, L\ p6, is also required. the ventilation portion of the cigarette in a small chamber The basis for the calculation of diluting air flow from and measures the flow into this chamber while a puff or pressure drop is the observation (3, 4, 6, 7) that pressure alternatively a constant flow is withdrawn from the ciga drop is essentially equal to the product of an impedance rette. The degree of air dilution is thus estimated from coefficient, a flow rate and a length (Darcy's Law). While the ratio of the volumetric flow rates. A second method is this relationship is nearly exact only for filter tips, it is that described by Reynolds and Wheeler (2), which en usable to a good approximation in tobacco columns where closes the cigarette except for the burning coal in an at a more complicated flow regime exists. -

Cigarette Brands Certified As Fire-Safe and Legal For
CIGARETTE BRANDS CERTIFIED AS FIRE-SAFE AND LEGAL FOR SALE IN VERMONT, APPROVED PACKAGE MARKINGS, AND RYO BRANDS LEGAL FOR SALE IN VERMONT LAST UPDATED September 19, 2018 Brands of cigarettes and roll-your-own tobacco not included in this combined listing may not be stamped or sold or offered for sale in Vermont. Any roll-your- own tobacco or cigarettes not on this combined listing are contraband and are subject to seizure and destruction. Sale or attempted sale of products not listed on this combined listing may also be punishable by license revocation and/or imposition of civil or criminal penalties. A. Cigarettes: This listing includes cigarettes for which both (a) the Attorney General has determined the manufacturer is in full compliance with 33 V.S.A.,Chapter 19, Subchapters 1A and 1B, and (b) the Department of Public Safety has approved fire-safe certifications and package marking pursuant to 20 V.S.A. §§ 2756 - 2757. Brand Style Flavor Filter Package Manufacturer 1839 Blue 100 Menthol Y Box U.S. Flue Cured Tob. Growers 1839 Blue 100 Y Box U.S. Flue Cured Tob. Growers 1839 Blue King Menthol Y Box U.S. Flue Cured Tob. Growers 1839 Blue King Y Box U.S. Flue Cured Tob. Growers 1839 Green 100 Menthol Y Box U.S. Flue Cured Tob. Growers 1839 Green King Menthol Y Box U.S. Flue Cured Tob. Growers 1839 King N Box U.S. Flue Cured Tob. Growers 1839 Red 100 Y Box U.S. Flue Cured Tob. Growers 1839 Red King Y Box U.S.