Effects of Insularity and Species Interactions Ec
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hybridization and Extinction in a Recent Passer Sparrow Zone
Hybridization and extinction in a recent Passer sparrow zone Vitalii Lichman Master of Science Thesis Department of Biosciences Faculty of Mathematics and Natural Sciences University of Oslo 01.11.2018 © Vitalii Lichman 2018 Hybridization and extinction in a recent Passer sparrow zone Vitalii Lichman http://www.duo.uio.no/ Trykk: Reprosentralen, Universitetet i Oslo Abstract The avifauna of Cape Verde archipelago is represented by three species within the Passer genus. Due to its distant localization from the continent and its variety of landscapes, this group of islands serves as objects of interest for studies in the sphere of evolutionary biology. From the beginning of the age of naturalistic explorations in the middle of 19th century, only few detailed ornithological expeditions were conducted until recently. In this connection, nowadays we have at our disposal only superficial information concerning the disposition of population structure and interspecific interactions within bird species, particularly sparrows. Technical progress and development of technologies in the field of molecular biology giving us an opportunity to investigate these processes more closely. This study clarifies phylogenetic relationships between 3 Passer species: 2 invasive (P. domesticus and P. hispaniolensis) and 1 endemic (P. Iagoensis). I also revealed a pronounced presence of P. hispaniolensis ancestry in P. domesticus genome that indicates existence of recent hybridization in the range of their contact, and supporting the notion that these species are prone to interspecific breeding elsewhere. I also found that P. Iagoensis has relatively high genome divergence - wide fixation index, that suggests absence of interbreeding between endemic and any of the invasive species. This is the first study of sparrows on the Cape Verde based on genetics and bioinformatics that presents explicit results on population structure Acknowledgements First and foremost, I would like to thank my supervisors Glenn-Peter Sætre and Mark Ravinet for all of their guidance. -

Italian Sparrows (Passer Italiae) Breeding in Black Kite (Milvus Migrans) Nests
Avocetta N° 15: 15-17 (1991) Italian Sparrows (Passer italiae) breeding in black kite (Milvus migrans) nests FRANCESCO PETRETTI Stazione Romana Osservazione e Protezione Uccelli Via degli Scipioni, 268/a 00192 Roma - ltaly Abstract - ltalian Sparrows were found breeding in Black Kite nests in a woodland in Centralltaly. The sparrows usually bred only in active raptor nests and their reproductive cycle seemed to be synchronized with that of the kites, the highest number of sparrow broods occurring when the raptor nests were occupied by the chicks. The adult sparrows were seen feeding in the raptor platforms when adult kites were away. Introduction check some sparrow nests and to collect data on their contents. This report illustrates breeding habits of Italian I made a complete survey of the woodland at least Sparrows (Passer italiae) in active Black Kite (Milvus twice during the breeding season, covering a grid of migrans) nests. transects spaced 50 metres apart, looking far House Sparrows (Passer domesticus) are known to sparrow nests. utilize the twig platforms made by raptors, storks and other large birds to build their domed nests (Summers-Smith 1988), but this behaviour has never Results been found associated exclusively with nest actively occupied by larger birds. I did not find Italian and Tree Sparrow nests in the In the present paper the Italian Sparrow is regarded woodland except those associated with the active as a true species, according to Johnston (1969). nests of the Black Kites. The closest Italian Sparrow pairs not associated with the kites were nesting in the farmland, at the edge of the woodland, on Study area and methods telephone poles and in concrete buildings. -

Songbird Remix Sparrows of the World
Avian Models for 3D Applications Characters and Texture Mapping by Ken Gilliland 1 Songbird ReMix Sparrows of the World Contents Manual Introduction 3 Overview and Use 3 Creating a Songbird ReMix Bird with Poser or DAZ Studio 4 One Folder to Rule Them All 4 Physical-based Rendering 5 Posing & Shaping Considerations 5 Where to Find Your Birds and Poses 6 Field Guide List of Species 7 Old World Sparrows Spanish Sparrow 8 Italian Sparrow 10 Eurasian Tree Sparrow 12 Dead Sea Sparrow 14 Arabian Golden Sparrow 16 Russet Sparrow 17 Cape Sparrow 19 Great Sparrow 21 Chestnut Sparrow 23 New World Sparrows American Tree Sparrow 25 Harris's Sparrow 28 Fox Sparrow 30 Golden-crowned Sparrow 32 Lark Sparrow 35 Lincoln's Sparrow 37 Rufous-crowned Sparrow 39 Savannah Sparrow 43 Rufous-winged Sparrow 47 Resources, Credits and Thanks 49 Copyrighted 2013-20 by Ken Gilliland www.songbirdremix.com Opinions expressed on this booklet are solely that of the author, Ken Gilliland, and may or may not reflect the opinions of the publisher. 2 Songbird ReMix Sparrows of the World Introduction Sparrows are probably the most familiar of all wild birds. Throughout history sparrows have been considered the harbinger of good or bad luck. They are referred to in many works of ancient literature and religious texts around the world. The ancient Egyptians used the sparrow symbol in their hieroglyphs to express evil tidings, the ancient Greeks associated it with Aphrodite, the goddess of love as a lustful messenger, and Jesus used sparrows as an example of divine providence in the Gospel of Matthew. -

AERC Wplist July 2015
AERC Western Palearctic list, July 2015 About the list: 1) The limits of the Western Palearctic region follow for convenience the limits defined in the “Birds of the Western Palearctic” (BWP) series (Oxford University Press). 2) The AERC WP list follows the systematics of Voous (1973; 1977a; 1977b) modified by the changes listed in the AERC TAC systematic recommendations published online on the AERC web site. For species not in Voous (a few introduced or accidental species) the default systematics is the IOC world bird list. 3) Only species either admitted into an "official" national list (for countries with a national avifaunistic commission or national rarities committee) or whose occurrence in the WP has been published in detail (description or photo and circumstances allowing review of the evidence, usually in a journal) have been admitted on the list. Category D species have not been admitted. 4) The information in the "remarks" column is by no mean exhaustive. It is aimed at providing some supporting information for the species whose status on the WP list is less well known than average. This is obviously a subjective criterion. Citation: Crochet P.-A., Joynt G. (2015). AERC list of Western Palearctic birds. July 2015 version. Available at http://www.aerc.eu/tac.html Families Voous sequence 2015 INTERNATIONAL ENGLISH NAME SCIENTIFIC NAME remarks changes since last edition ORDER STRUTHIONIFORMES OSTRICHES Family Struthionidae Ostrich Struthio camelus ORDER ANSERIFORMES DUCKS, GEESE, SWANS Family Anatidae Fulvous Whistling Duck Dendrocygna bicolor cat. A/D in Morocco (flock of 11-12 suggesting natural vagrancy, hence accepted here) Lesser Whistling Duck Dendrocygna javanica cat. -

The Mysterious Bird Outbreak of 1779 in Southeastern Iberian Peninsula: a Massive Irruption of the Spanish Sparrow Passer Hispaniolensis from Africa?
Animal Biodiversity and Conservation 41.2 (2018) 365 The mysterious bird outbreak of 1779 in southeastern Iberian peninsula: a massive irruption of the Spanish sparrow Passer hispaniolensis from Africa? J. J. Ferrero–García, L. M. Torres–Vila, P. P. Bueno Ferrero–García, J. J., Torres–Vila, L. M., Bueno, P. P., 2018. The mysterious bird outbreak of 1779 in southeast- ern Iberian peninsula: a massive irruption of the Spanish sparrow Passer hispaniolensis from Africa? Animal Biodiversity and Conservation, 41.2: 365–377, Doi: https://doi.org/10.32800/abc.2018.41.0365. Abstract The mysterious bird outbreak of 1779 in southeastern Iberian peninsula: a massive irruption of the Spanish sparrow Passer hispaniolensis from Africa? Several current and past bibliographical references mention the sudden pest outbreak of a mysterious sparrow–like bird in the southeastern Iberian peninsula in 1779. Based on these references, we investigated unpublished documentary sources from various historical archives that reflected the actions carried out by public authorities against the bird pest. Some narratives come from direct witnesses who sometimes provided relevant data on the origin and biology of the birds involved. From the analysis and interpretation of these data, it was clear that the bird outbreak was caused by an unusual pas- serine in southeastern Iberia. In May 1779, birds irrupted in large numbers into several localities in the current provinces of Alicante, Murcia and Almería, probably coming from North Africa. Damage caused to cereal crops was meaningful and the extraordinary alarm generated in the people motivated the intervention of both local authorities and government institutions. The birds formed large arboreal colonies, building multiple nests per tree. -
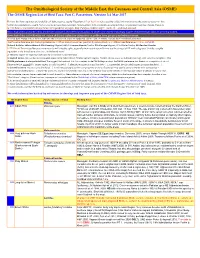
OSME List V3.4 Passerines-2
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa: Part C, Passerines. Version 3.4 Mar 2017 For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates either added information since the previous version or that further documentation is sought. Not all synonyms have been examined. Serial numbers (SN) are merely an administrative conveninence and may change. Please do not cite them as row numbers in any formal correspondence or papers. Key: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. The Passerine Reference List (including References for Hypothetical passerines [see Part E] and explanations of Abbreviated References) follows at Part D. Notes↓ & Status abbreviations→ BM=Breeding Migrant, SB/SV=Summer Breeder/Visitor, PM=Passage Migrant, WV=Winter Visitor, RB=Resident Breeder 1. PT=Parent Taxon (used because many records will antedate splits, especially from recent research) – we use the concept of PT with a degree of latitude, roughly equivalent to the formal term sensu lato , ‘in the broad sense’. 2. The term 'report' or ‘reported’ indicates the occurrence is unconfirmed. -

EUROPEAN BIRDS of CONSERVATION CONCERN Populations, Trends and National Responsibilities
EUROPEAN BIRDS OF CONSERVATION CONCERN Populations, trends and national responsibilities COMPILED BY ANNA STANEVA AND IAN BURFIELD WITH SPONSORSHIP FROM CONTENTS Introduction 4 86 ITALY References 9 89 KOSOVO ALBANIA 10 92 LATVIA ANDORRA 14 95 LIECHTENSTEIN ARMENIA 16 97 LITHUANIA AUSTRIA 19 100 LUXEMBOURG AZERBAIJAN 22 102 MACEDONIA BELARUS 26 105 MALTA BELGIUM 29 107 MOLDOVA BOSNIA AND HERZEGOVINA 32 110 MONTENEGRO BULGARIA 35 113 NETHERLANDS CROATIA 39 116 NORWAY CYPRUS 42 119 POLAND CZECH REPUBLIC 45 122 PORTUGAL DENMARK 48 125 ROMANIA ESTONIA 51 128 RUSSIA BirdLife Europe and Central Asia is a partnership of 48 national conservation organisations and a leader in bird conservation. Our unique local to global FAROE ISLANDS DENMARK 54 132 SERBIA approach enables us to deliver high impact and long term conservation for the beneit of nature and people. BirdLife Europe and Central Asia is one of FINLAND 56 135 SLOVAKIA the six regional secretariats that compose BirdLife International. Based in Brus- sels, it supports the European and Central Asian Partnership and is present FRANCE 60 138 SLOVENIA in 47 countries including all EU Member States. With more than 4,100 staf in Europe, two million members and tens of thousands of skilled volunteers, GEORGIA 64 141 SPAIN BirdLife Europe and Central Asia, together with its national partners, owns or manages more than 6,000 nature sites totaling 320,000 hectares. GERMANY 67 145 SWEDEN GIBRALTAR UNITED KINGDOM 71 148 SWITZERLAND GREECE 72 151 TURKEY GREENLAND DENMARK 76 155 UKRAINE HUNGARY 78 159 UNITED KINGDOM ICELAND 81 162 European population sizes and trends STICHTING BIRDLIFE EUROPE GRATEFULLY ACKNOWLEDGES FINANCIAL SUPPORT FROM THE EUROPEAN COMMISSION. -

Supplementary Material
Passer hispaniolensis (Spanish Sparrow) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 4 Sources of reported national population data p. 6 Species factsheet bibliography p. 9 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Passer hispaniolensis (Spanish Sparrow) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population -
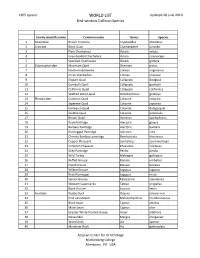
WORLD LIST Updated 28 June 2019 Bird-Window Collision Species
1305 species WORLD LIST Updated 28 June 2019 Bird-window Collision Species Family scientific name Common name Genus Species 1 Tinamidae Brown Tinamou Crypturellus obsoletus 2 Cracidae Black Guan Chamaepetes unicolor 3 Plain Chachalaca Ortalis vetula 4 Grey-headed Chachalaca Ortalis cinereiceps 5 Speckled Chachalaca Ortalis guttata 6 Odontophoridae Mountain Quail Oreortyx pictus 7 Northern Bobwhite Colinus virginianus 8 Crested Bobwhite Colinus cristatus 9 Elegant Quail Callipepla douglasii 10 Gambel's Quail Callipepla gambelii 11 California Quail Callipepla californica 12 Spotted Wood-quail Odontophorus guttatus 13 Phasianidae Common Quail Coturnix coturnix 14 Japanese Quail Coturnix japonica 15 Harlequin Quail Coturnix delegorguei 16 Stubble Quail Coturnix pectoralis 17 Brown Quail Synoicus ypsilophorus 18 Rock Partridge Alectoris graeca 19 Barbary Partridge Alectoris barbara 20 Red-legged Partridge Alectoris rufa 21 Chinese Bamboo-partridge Bambusicola thoracicus 22 Copper Pheasant Syrmaticus soemmerringii 23 Common Pheasant Phasianus colchicus 24 Grey Partridge Perdix perdix 25 Wild Turkey Meleagris gallopavo 26 Ruffed Grouse Bonasa umbellus 27 Hazel Grouse Bonasa bonasia 28 Willow Grouse Lagopus lagopus 29 Rock Ptarmigan Lagopus muta 30 Spruce Grouse Falcipennis canadensis 31 Western Capercaillie Tetrao urogallus 32 Black Grouse Lyrurus tetrix 33 Anatidae Ruddy Duck Oxyura jamaicensis 34 Pink-eared Duck Malacorhynchus membranaceus 35 Black Swan Cygnus atratus 36 Mute Swan Cygnus olor 37 Greater White-fronted Goose Anser albifrons 38 -

Download Download
Biodiversity Observations http://bo.adu.org.za An electronic journal published by the Animal Demography Unit at the University of Cape Town The scope of Biodiversity Observations consists of papers describing observations about biodiversity in general, including animals, plants, algae and fungi. This includes observations of behaviour, breeding and flowering patterns, distributions and range extensions, foraging, food, movement, measurements, habitat and colouration/plumage variations. Biotic interactions such as pollination, fruit dispersal, herbivory and predation fall within the scope, as well as the use of indigenous and exotic species by humans. Observations of naturalised plants and animals will also be considered. Biodiversity Observations will also publish a variety of other interesting or relevant biodiversity material: reports of projects and conferences, annotated checklists for a site or region, specialist bibliographies, book reviews and any other appropriate material. Further details and guidelines to authors are on this website. Lead Editor: Arnold van der Westhuizen – Paper Editor: Amour McCarthy and H.D. Oschadleus TIP OF THE ICEBERG: HYBRID CAPE SPARROW AND GREAT SPARROW IN NAMIBIA Ursula Franke-Bryson Recommended citation format: Franke-Bryson U 2016. Tip of the iceberg: hybrid Cape Sparrow and Great Sparrow in Namibia. Biodiversity Observations 7.82: 1–6 URL: http://bo.adu.org.za/content.php?id=275 Published online: 21 November 2016 – ISSN 2219-0341 – Biodiversity Observations 7.82: 1-6 1 AFRING NEWS In December 2014 -

Taxonomy of House Sparrows and Their Allies in the Mediterranean Basin
TAXONOMY OF HOUSE SPARROWS AND THEIR ALLIES IN THE MEDITERRANEAN BASIN RICHARD F. JOHNSTON Museum of Natural History and Department of Zoology The University of Kansas Lawrence, Kansas 66044 Approximately 3606 years ago there occurred the biology of these sparrows is that geograph- a significant movement of northern European ically disjunct populations may be phenetically agricultural man into southern European and much alike. This has suggested that they may Mediterranean regions (establishment of the also be genetically similar, but experimental Lake Dwellers in northern Italy, see Brea 1946 verification of this hypothesis is lacking. It is et seq.). Seemingly also with man came his known that the phenotypic feather coat of the newly-acquired commensal, the House Sparrow sparrows has a highly polygenic basis (see be- (Passer domesticus), which had been living low), and it is thus entirely possible that with man in northern Europe since its pre- similar phenotypes can have different geno- sumptive origins in the Near East, perhaps types, and vice versa. some 5500 years earlier. Indigenous sparrows Polygenic control of gross phenotype may in Italy, probably also commensal in 1600 BC well be the expected condition, but we need and almost certainly Willow Sparrows (con- not merely assume it for Mediterranean spar- ventionally considered a species by most tax- rows; Macke (1965; see also table 7) has shown onomists as Passer hispaniolmwis), shared a that the F1 of the cross, House Sparrow x common ancestry with House Sparrows but Willow Sparrow, may include a sampling of were phenotypically distinct. Interbreeding of nearly the entire range of intermediate pheno- the two phenetic kinds ensued, followed by types, hence of genotypes. -

Studies of West Palearctic Birds 197
Studies of West Palearctic birds 197. Tree Sparrow J. Denis Summers-Smith ABSTRACT The Tree Sparrow Passer montanus and the House Sparrow P. domestkus are the only two members of the genus Passer that breed in Britain & Ireland. They are an interesting pair of congeners: both have extensive distributions in the Palearctic and Oriental Regions; both are commensals of Man in at least parts of their ranges; and both, despite some recent decreases in numbers in areas where they have long been established, are still expanding their ranges. A recent article in this series concentrated on the House Sparrow (Summers-Smith 1994); we now turn to the less familiar of the British pair, the Tree Sparrow. It is an odd comment on human nature that most British birdwatchers, having ticked off the House Sparrow on 1st January for their annual list, scarcely give the species another look; yet the Tree Sparrow is generally regarded with great affection and faithfully recorded throughout the year. I do not think that this is solely because the Tree Sparrow tends to be regarded as something of a rarity in Britain; there is something appealing about it that makes it particularly attractive to those interested in birds. The fact that the Tree Sparrow is a frequent subject in Oriental painting, going back as far as the twelfth century, may perhaps reflect something of this appeal. The simple brush strokes of the Chinese and Japanese styles of painting show an obvious familiarity with the bird and its behaviour. There may, however, be another side to the fascination in the East.