VIRAL HEPATITIS Poljak Mario
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Communicable Disease Chart
COMMON INFECTIOUS ILLNESSES From birth to age 18 Disease, illness or organism Incubation period How is it spread? When is a child most contagious? When can a child return to the Report to county How to prevent spreading infection (management of conditions)*** (How long after childcare center or school? health department* contact does illness develop?) To prevent the spread of organisms associated with common infections, practice frequent hand hygiene, cover mouth and nose when coughing and sneezing, and stay up to date with immunizations. Bronchiolitis, bronchitis, Variable Contact with droplets from nose, eyes or Variable, often from the day before No restriction unless child has fever, NO common cold, croup, mouth of infected person; some viruses can symptoms begin to 5 days after onset or is too uncomfortable, fatigued ear infection, pneumonia, live on surfaces (toys, tissues, doorknobs) or ill to participate in activities sinus infection and most for several hours (center unable to accommodate sore throats (respiratory diseases child’s increased need for comfort caused by many different viruses and rest) and occasionally bacteria) Cold sore 2 days to 2 weeks Direct contact with infected lesions or oral While lesions are present When active lesions are no longer NO Avoid kissing and sharing drinks or utensils. (Herpes simplex virus) secretions (drooling, kissing, thumb sucking) present in children who do not have control of oral secretions (drooling); no exclusions for other children Conjunctivitis Variable, usually 24 to Highly contagious; -

Hepatitis Virus in Long-Fingered Bats, Myanmar
DISPATCHES Myanmar; the counties are adjacent to Yunnan Province, Hepatitis Virus People’s Republic of China. The bats covered 6 species: Miniopterus fuliginosus (n = 640), Hipposideros armiger in Long-Fingered (n = 8), Rhinolophus ferrumequinum (n = 176), Myotis chi- nensis (n = 11), Megaderma lyra (n = 6), and Hipposideros Bats, Myanmar fulvus (n = 12). All bat tissue samples were subjected to vi- Biao He,1 Quanshui Fan,1 Fanli Yang, ral metagenomic analysis (unpublished data). The sampling Tingsong Hu, Wei Qiu, Ye Feng, Zuosheng Li, of bats for this study was approved by the Administrative Yingying Li, Fuqiang Zhang, Huancheng Guo, Committee on Animal Welfare of the Institute of Military Xiaohuan Zou, and Changchun Tu Veterinary, Academy of Military Medical Sciences, China. We used PCR to further study the prevalence of or- During an analysis of the virome of bats from Myanmar, thohepadnavirus in the 6 bat species; the condition of the a large number of reads were annotated to orthohepadnavi- samples made serologic assay and pathology impracticable. ruses. We present the full genome sequence and a morpho- Viral DNA was extracted from liver tissue of each of the logical analysis of an orthohepadnavirus circulating in bats. 853 bats by using the QIAamp DNA Mini Kit (QIAGEN, This virus is substantially different from currently known Hilden, Germany). To detect virus in the samples, we con- members of the genus Orthohepadnavirus and represents ducted PCR by using the TaKaRa PCR Kit (TaKaRa, Da- a new species. lian, China) with a pair of degenerate pan-orthohepadnavi- rus primers (sequences available upon request). The PCR he family Hepadnaviridae comprises 2 genera (Ortho- reaction was as follows: 45 cycles of denaturation at 94°C Thepadnavirus and Avihepadnavirus), and viruses clas- for 30 s, annealing at 54°C for 30 s, extension at 72°C for sified within these genera have a narrow host range. -

Virus Particle Structures
Virus Particle Structures Virus Particle Structures Palmenberg, A.C. and Sgro, J.-Y. COLOR PLATE LEGENDS These color plates depict the relative sizes and comparative virion structures of multiple types of viruses. The renderings are based on data from published atomic coordinates as determined by X-ray crystallography. The international online repository for 3D coordinates is the Protein Databank (www.rcsb.org/pdb/), maintained by the Research Collaboratory for Structural Bioinformatics (RCSB). The VIPER web site (mmtsb.scripps.edu/viper), maintains a parallel collection of PDB coordinates for icosahedral viruses and additionally offers a version of each data file permuted into the same relative 3D orientation (Reddy, V., Natarajan, P., Okerberg, B., Li, K., Damodaran, K., Morton, R., Brooks, C. and Johnson, J. (2001). J. Virol., 75, 11943-11947). VIPER also contains an excellent repository of instructional materials pertaining to icosahedral symmetry and viral structures. All images presented here, except for the filamentous viruses, used the standard VIPER orientation along the icosahedral 2-fold axis. With the exception of Plate 3 as described below, these images were generated from their atomic coordinates using a novel radial depth-cue colorization technique and the program Rasmol (Sayle, R.A., Milner-White, E.J. (1995). RASMOL: biomolecular graphics for all. Trends Biochem Sci., 20, 374-376). First, the Temperature Factor column for every atom in a PDB coordinate file was edited to record a measure of the radial distance from the virion center. The files were rendered using the Rasmol spacefill menu, with specular and shadow options according to the Van de Waals radius of each atom. -

Mumps Virus Pathogenesis Clinical Features
Mumps Mumps Mumps is an acute viral illness. Parotitis and orchitis were described by Hippocrates in the 5th century BCE. In 1934, Johnson and Goodpasture showed that mumps could be transmitted from infected patients to rhesus monkeys and demonstrated that mumps was caused by a filterable agent present in saliva. This agent was later shown to be a virus. Mumps was a frequent cause of outbreaks among military personnel in the prevaccine era, and was one of the most common causes of aseptic meningitis and sensorineural deafness in childhood. During World War I, only influenza and gonorrhea were more common causes of hospitalization among soldiers. Outbreaks of mumps have been reported among military personnel as recently as 1986. Mumps Virus Mumps virus is a paramyxovirus in the same group as parainfluenza and Newcastle disease virus. Parainfluenza and Newcastle disease viruses produce antibodies that cross- 11 react with mumps virus. The virus has a single-stranded RNA genome. The virus can be isolated or propagated in cultures of various human and monkey tissues and in embryonated eggs. It has been recovered from the saliva, cerebrospinal fluid, urine, blood, milk, and infected tissues of patients with mumps. Mumps virus is rapidly inactivated by formalin, ether, chloroform, heat, and ultraviolet light. Pathogenesis The virus is acquired by respiratory droplets. It replicates in the nasopharynx and regional lymph nodes. After 12–25 days a viremia occurs, which lasts from 3 to 5 days. During the viremia, the virus spreads to multiple tissues, including the meninges, and glands such as the salivary, pancreas, testes, and ovaries. -

Discovery of a Highly Divergent Hepadnavirus in Shrews from China
Virology 531 (2019) 162–170 Contents lists available at ScienceDirect Virology journal homepage: www.elsevier.com/locate/virology Discovery of a highly divergent hepadnavirus in shrews from China T Fang-Yuan Niea,b,1, Jun-Hua Tianc,1, Xian-Dan Lind,1, Bin Yuc, Jian-Guang Xinge, Jian-Hai Caof, ⁎ ⁎⁎ Edward C. Holmesg,h, Runlin Z. Maa, , Yong-Zhen Zhangb,h, a State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China b State Key Laboratory for Infectious Disease Prevention and Control; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases; Department of Zoonoses, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Changping, Beijing, China c Wuhan Center for Disease Control and Prevention, Wuhan, China d Wenzhou Center for Disease Control and Prevention, Wenzhou, Zhejiang Province, China e Wencheng Center for Disease Control and Prevention, Wencheng, Zhejiang Province, China f Longwan Center for Disease Control and Prevention, Longwan District, Wenzhou, Zhejiang Province, China g Marie Bashir Institute for Infectious Diseases and Biosecurity, Charles Perkins Centre, School of Life and Environmental Sciences and Sydney Medical School, The University of Sydney, Sydney, NSW 2006, Australia h Shanghai Public Health Clinical Center & Institute of Biomedical Sciences, Fudan University, Shanghai, China ARTICLE INFO ABSTRACT Keywords: Limited sampling means that relatively little is known about the diversity and evolutionary history of mam- Hepadnaviruses malian members of the Hepadnaviridae (genus Orthohepadnavirus). An important case in point are shrews, the Shrews fourth largest group of mammals, but for which there is limited knowledge on the role they play in viral evo- Phylogeny lution and emergence. -

And Giant Guitarfish (Rhynchobatus Djiddensis)
VIRAL DISCOVERY IN BLUEGILL SUNFISH (LEPOMIS MACROCHIRUS) AND GIANT GUITARFISH (RHYNCHOBATUS DJIDDENSIS) BY HISTOPATHOLOGY EVALUATION, METAGENOMIC ANALYSIS AND NEXT GENERATION SEQUENCING by JENNIFER ANNE DILL (Under the Direction of Alvin Camus) ABSTRACT The rapid growth of aquaculture production and international trade in live fish has led to the emergence of many new diseases. The introduction of novel disease agents can result in significant economic losses, as well as threats to vulnerable wild fish populations. Losses are often exacerbated by a lack of agent identification, delay in the development of diagnostic tools and poor knowledge of host range and susceptibility. Examples in bluegill sunfish (Lepomis macrochirus) and the giant guitarfish (Rhynchobatus djiddensis) will be discussed here. Bluegill are popular freshwater game fish, native to eastern North America, living in shallow lakes, ponds, and slow moving waterways. Bluegill experiencing epizootics of proliferative lip and skin lesions, characterized by epidermal hyperplasia, papillomas, and rarely squamous cell carcinoma, were investigated in two isolated poopulations. Next generation genomic sequencing revealed partial DNA sequences of an endogenous retrovirus and the entire circular genome of a novel hepadnavirus. Giant Guitarfish, a rajiform elasmobranch listed as ‘vulnerable’ on the IUCN Red List, are found in the tropical Western Indian Ocean. Proliferative skin lesions were observed on the ventrum and caudal fin of a juvenile male quarantined at a public aquarium following international shipment. Histologically, lesions consisted of papillomatous epidermal hyperplasia with myriad large, amphophilic, intranuclear inclusions. Deep sequencing and metagenomic analysis produced the complete genomes of two novel DNA viruses, a typical polyomavirus and a second unclassified virus with a 20 kb genome tentatively named Colossomavirus. -

Measles, Mumps, and Rubella
Measles, Mumps, and Rubella Developed by Vini Vijayan, MD, in collaboration with the ANGELS Team. Last reviewed by Vini Vijayan, MD, January 24, 2017. Because measles, mumps, and rubella can be prevented by a combination vaccine, all 3 of these illnesses are discussed in this Guideline. The following vaccines are available in the United States: Live measles, mumps, and rubella virus vaccine (MMR) Live measles, mumps, rubella, and varicella virus vaccine (MMRV) MEASLES (RUBEOLA) Key Points Measles, also called rubeola or red measles, is a highly contagious viral illness characterized by fever, malaise, rash, cough, coryza (runny nose), and conjunctivitis. Measles is a leading cause of morbidity and mortality in developing countries. Over the past decade measles has been making a resurgence in the United States. Vaccination with MMR or MMRV is highly effective in preventing the disease. Introduction The Virus The measles virus is a single-stranded, enveloped ribonucleic acid (RNA) virus of the genus Morbillivirus within the family Paramyxoviridae. 1 The virus enters the respiratory epithelium of the nasopharynx and regional lymph nodes resulting in viremia and dissemination to the skin, respiratory tract, and other organs. The peak incidence of measles in temperate areas is late winter and early spring. Transmission Measles is a highly contagious virus with an estimated 90% secondary infection rate in susceptible domestic contacts. Measles is spread by droplets from respiratory secretions and close personal contact or direct contact with infected nasal or throat secretions. Measles virus can be transmitted by an infected person from 4 days prior to the onset of the rash to 4 days after the rash erupts. -
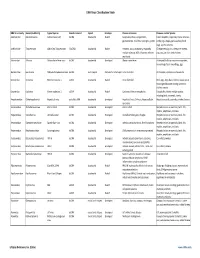
Virus Classification Tables V2.Vd.Xlsx
DNA Virus Classification Table DNA Virus Family Genera (Subfamily) Typical Species Genetic material Capsid Envelope Disease in Humans Diseases in other Species Adenoviridae Mastadenovirus Adenoviruses 1‐47 dsDNA Icosahedral Naked Respiratory illness; conjunctivitis, Canine hepatitis, respiratory illness in horses, gastroenteritis, tonsillitis, meningitis, cystitis cattle, pigs, sheep, goats, sea lions, birds dogs, squirrel enteritis Anelloviridae Torqueviruses Alpha‐Zeta Torqueviruses (‐)ssDNA Icosahedral Naked Hepatitis, lupus, pulmonary, myopathy, Chimpanzee, pig, cow, sheep, tree shrews, multiple sclerosis; 90% of humans infected pigs, cats, sea lions and chickens worldwide Asfarviridae Asfivirus African Swine fever virus dsDNA Icosahedral Enveloped African swine fever Arthropod (tick) transmission or ingestion; hemorrhagic fever in warthogs, pigs Baculoviridae Baculovirus Alpha‐Gamma Baculoviruses dsDNA Stick shaped Occluded or Enveloped none identified Arthropods, Lepidoptera, crustaceans Circoviridae Circovirus Porcine circovirus 1 ssDNA Icosahedral Naked none identified Birds, pigs, dogs; bats; rodents; causes post‐ weaning multisystem wasting syndrome, chicken anemia Circoviridae Cyclovirus Human cyclovirus 1 ssDNA Icosahedral Naked Cyclovirus Vietnam encephalitis Encephalitis; infects multiple species including birds, mammals, insects Hepadnaviridae Orthohepadnavirus Hepatitis B virus partially ssDNA Icosahedral Enveloped Hepatitis B virus; Cirrhosis, Hepatocellular Hepatitis in ducks, squirrels, primates, herons carcinoma Herpesviridae -

Viruses in Transplantation - Not Always Enemies
Viruses in transplantation - not always enemies Virome and transplantation ECCMID 2018 - Madrid Prof. Laurent Kaiser Head Division of Infectious Diseases Laboratory of Virology Geneva Center for Emerging Viral Diseases University Hospital of Geneva ESCMID eLibrary © by author Conflict of interest None ESCMID eLibrary © by author The human virome: definition? Repertoire of viruses found on the surface of/inside any body fluid/tissue • Eukaryotic DNA and RNA viruses • Prokaryotic DNA and RNA viruses (phages) 25 • The “main” viral community (up to 10 bacteriophages in humans) Haynes M. 2011, Metagenomic of the human body • Endogenous viral elements integrated into host chromosomes (8% of the human genome) • NGS is shaping the definition Rascovan N et al. Annu Rev Microbiol 2016;70:125-41 Popgeorgiev N et al. Intervirology 2013;56:395-412 Norman JM et al. Cell 2015;160:447-60 ESCMID eLibraryFoxman EF et al. Nat Rev Microbiol 2011;9:254-64 © by author Viruses routinely known to cause diseases (non exhaustive) Upper resp./oropharyngeal HSV 1 Influenza CNS Mumps virus Rhinovirus JC virus RSV Eye Herpes viruses Parainfluenza HSV Measles Coronavirus Adenovirus LCM virus Cytomegalovirus Flaviviruses Rabies HHV6 Poliovirus Heart Lower respiratory HTLV-1 Coxsackie B virus Rhinoviruses Parainfluenza virus HIV Coronaviruses Respiratory syncytial virus Parainfluenza virus Adenovirus Respiratory syncytial virus Coronaviruses Gastro-intestinal Influenza virus type A and B Human Bocavirus 1 Adenovirus Hepatitis virus type A, B, C, D, E Those that cause -

Infection Prevention News & Updates
APRIL 2018 INFECTION PREVENTION NEWS & UPDATES FAST FACTS HEPATITIS A Hepatitis A outbreaks continue to occur as the global prevalence appears Severity to be rapidly increasing. Victoria Australia is seeing its outbreak grow with MULTI-COUNTRY OUTBREAK 58 confirmed cases, 7 probably cases, 16 cases under early investigation, and one death. Being homeless or an IV drug user is associated with a higher probability of becoming infected. The initial laboratory analysis suggests the strain is similar to a strain circulating in Europe, suggesting Transmission CONTACT there is a travel associated case linking the outbreaks. The outbreak in Kentucky in the US is continuing to grow with 150 cases, 124 of which are in the greater Louisville area. As with other outbreaks, the homeless and IV drug users are at a higher risk of getting disease. The Utah outbreak has 217 confirmed cases. Genetic sequencing of the virus has shown that the Arizona and California outbreaks reported previously are tied to these outbreaks as well. Location AUSTRALIA, USA Hepatitis A is an infection that causes an inflammation of the liver. There are a number of viruses that can cause Hepatitis including Hepatitis A, Hepatitis B, and Hepatitis C, with vaccinations available for Hepatitis A and Hepatitis B. Hepatitis A is passed to other people through contact with infected feces, or if an infected person touches objects after using the toilet and not washing their hands, including contaminating food or drink from contact with contaminated hands, so handwashing and surface disinfection are important interventions to prevent the spread of the virus. It can also be spread by sexual contact. -

Perspectives on Vaccination Against Respiratory Syncytial Virus
Perspectives on vaccination against respiratory syncytial virus What makes the development of RSV vaccines challenging? Oliver Wicht PhD, Projectleader MD-RSV antibodies RIVM, Centrum infektieziektenbestrijding http://www.strategischprogramma rivm.nl/gezondheid_afweer RSV is a pleomorphic paramyxovirus Jeffree et al. Virology, Volume 306, Issue 2, 2003, 254–267 ● Same family as measles virus, mumps virus, and metapneumovirus ● Vaccine is not available ● Pathogenesis varies from mild cold to bronchiolitis and pneumonia, rarely lethal ● reinfections frequently occur throughout life, 5-20% of population per annum 3 Perspectives on vaccination against RSV | 18-11-2015 RSV-mediated respiratory disease RSV infection by large droplets and contaminated surfaces virus shedding URT virus cleared 3- 5 days 1- 3 weeks Upper respiratory tract infection Rhinorrhea, cough, common cold 4 Perspectives on vaccination against RSV | 18-11-2015 RSV-mediated respiratory disease RSV infection by large droplets and Lower respiratory tract infection: contaminated surfaces Fever, malaise, headache, myalgia, sore throat, cough, dyspnea, rhinorrhea Otits, Sinositis, brochiolitis, pneumonia 1- 3 days virus shedding 4 - 8 months virus LRT URT virus cleared cleared 3- 5 days 1- 3 weeks possible longer term effects: airway hyperreactivity, wheezing, asthma 5 Perspectives on vaccination against RSV | 18-11-2015 RSV-mediated respiratory disease ● RSV stays in the lungs, usually not systemic – Mucosal pathogens are hard to study because conditions are hard to mimick in -

The Approved List of Biological Agents Advisory Committee on Dangerous Pathogens Health and Safety Executive
The Approved List of biological agents Advisory Committee on Dangerous Pathogens Health and Safety Executive © Crown copyright 2021 First published 2000 Second edition 2004 Third edition 2013 Fourth edition 2021 You may reuse this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence. To view the licence visit www.nationalarchives.gov.uk/doc/ open-government-licence/, write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email [email protected]. Some images and illustrations may not be owned by the Crown so cannot be reproduced without permission of the copyright owner. Enquiries should be sent to [email protected]. The Control of Substances Hazardous to Health Regulations 2002 refer to an ‘approved classification of a biological agent’, which means the classification of that agent approved by the Health and Safety Executive (HSE). This list is approved by HSE for that purpose. This edition of the Approved List has effect from 12 July 2021. On that date the previous edition of the list approved by the Health and Safety Executive on the 1 July 2013 will cease to have effect. This list will be reviewed periodically, the next review is due in February 2022. The Advisory Committee on Dangerous Pathogens (ACDP) prepares the Approved List included in this publication. ACDP advises HSE, and Ministers for the Department of Health and Social Care and the Department for the Environment, Food & Rural Affairs and their counterparts under devolution in Scotland, Wales & Northern Ireland, as required, on all aspects of hazards and risks to workers and others from exposure to pathogens.